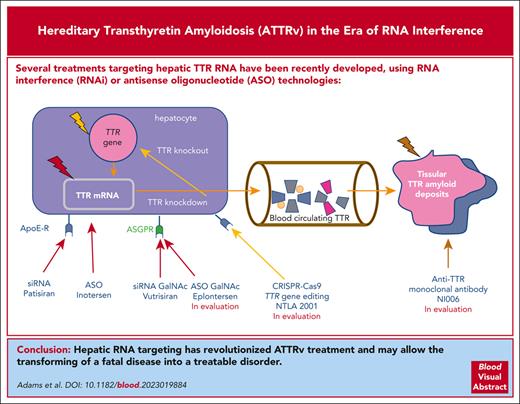
Abstract
Hereditary transthyretin amyloidosis (ATTRv) is a rare autosomal dominant adult-onset disorder caused by point mutations in the transthyretin (TTR) gene encoding TTR, also known as prealbumin. ATTRv survival ranges from 3 to 10 years, and peripheral nervous system and heart are usually the 2 main tissues affected, although central nervous system and eye may also be involved. Because the liver is the main TTR protein secretor organ, it has been the main target of treatments developed these last years, including liver transplantation, which has been shown to significantly increase survival in a subset of patients carrying the so-called “early-onset Val30Met” TTR gene mutation. More recently, treatments targeting hepatic TTR RNA have been developed. Hepatic TTR RNA targeting is performed using RNA interference (RNAi) and antisense oligonucleotide (ASO) technologies involving lipid nanoparticle carriers or N-acetylgalactosamine fragments. RNAi and ASO treatments induce an 80% decrease in TTR liver production for a period of 1 to 12 weeks. ASO and RNAi phase 3 trials in patients with TTR-related polyneuropathy have shown a positive impact on neuropathy clinical scores and quality of life end points, and delayed RNAi treatment negatively affects survival. Clinical trials specifically investigating RNAi therapy in TTR cardiomyopathy are underway. Hepatic RNA targeting has revolutionized ATTRv treatment and may allow for the transforming a fatal disease into a treatable disorder. Because retina and choroid plexus secrete limited quantities of TTR protein, both tissues are now seen as the next targets for fully controlling the disease.
Introduction
Hereditary transthyretin amyloidosis (ATTRv) is a rare adult-onset disorder resulting from transthyretin (TTR) gene autosomal dominant point mutations and manifesting with various phenotypes.1 Transthyretin is produced mainly by the liver but also by choroid plexuses and retinal pigment epithelium.2 ATTRv is a progressive and disabling disorder with a 2.5- to 10-year survival range after diagnosis.3-5 The main phenotypes are peripheral neuropathy, including somatic and autonomic nerves (ATTRv-PN) and cardiac amyloidosis (ATTRv-CM). ATTRv may also present with leptomeningeal6 and vitreous amyloidosis.7 ATTRv amyloidosis treatment benefited from major advances in the past 30 years, including TTR gene sequencing for the detection of the currently known 140 amyloidogenic TTR variants,8 mini-invasive biopsy for the detection of amyloid deposits,9 and bone scintigraphy for the detection of cardiac TTR uptake.10 The first disease-modifying therapy developed for ATTRv was liver transplantation (LT), which, by removing the main source of variant TTR, stopped disease progression and doubled survival time in patients with the so-called “early-onset V30M ATTRv-PN (EOV30M)” mutation.11 TTR stabilizer tafamidis was shown to stop disease progression in patients with EOV30M12 and improved survival for those with ATTRv-CM13; particularly, in ATTR wild-type (ATTRwt), TTR stabilizer diflunisal slowed progression in various ATTRv-PNs.14 LT limited the effect in patients with late onset (LO) ATTRV30M-PN (LOV30M), and other TTR gene variants demonstrated the role of wtTTR in the pathophysiology of the disease. Indeed, amyloidosis continued to progress because of continued wtTTR accumulation. In this context, RNA targeting was developed to stop hepatocyte production of both variant TTR and wtTTR. In this review, we report the results of successful RNA targeting phase 3 trials, including RNA interference (RNAi) and antisense oligonucleotides (ASO) in ATTRv-PN, and ongoing clinical trials for ATTRv-CM.
Presentation of the disease
Hereditary transthyretin amyloidosis are rare autosomal dominant adult-onset disorders resulting from TTR gene point mutations and resulting in peripheral neuropathy, cardiomyopathy, and ocular and central nervous system (CNS) manifestations (Table 1).
ATTRv phenotypes
| . | ATTRv-PN . | ATTRv-CM . | ATTRv-LM or CNS . | ATTRv-O . | |
|---|---|---|---|---|---|
| Epidemiology | 10 000 | Main variant: V122I: 3.4% of African Americans 40 000 gene carriers Other variants in many countries | 82 cases reported (global) | Ubiquitary (dozens of families) | |
| No. of TTR variants | 100 | 24 | 15 | 5 | |
| Main variants | EO V30M | LO V30M, S77Y, E89Q, and I107V | V122I, T60A, I68L, and L111M | D18G, Y69H V30G, L12P, A25T, V30M, G53E, and Y114C | G83A, R34G, Y69H, K35T, and W41L |
| Age at onset, y | 31.9 ± 7.6 | 64.5 ± 6.5 | 74 ± 7 (rare before 50 y) | 44.9 ± 9.1 | 35-56 |
| Male sex, % | 50 | 86 | 80-85 | Unk | Unk |
| Ethnicity | Portuguese Japanese Brazilian Mallorcan Cypriot | Global | V122I: African Americans and Caribbean Other variants: clusters | Various | Various |
| Duration of symptoms at diagnosis (y) | 2 | 2.8 | 1 | Unk | Unk |
| Main clinical manifestations | Symptoms in the feet Autonomic symptoms Unexplained weight loss | Neuropathic (80%) Autonomic (10%) Cardiac (4%-20%) | Heart failure symptoms Fluid overload History of CTS 50% | Cognitive impairment Ataxia Headache Seizures Stroke SAH Hearing loss | Blurred vision Vitreous opacities Glaucoma |
| Penetrance | High 80% at 50 y | High 80% at 80 y | Low | ||
| Tools for diagnosis | TTR gene sequencing Skin biopsy and LSGB | TTR gene sequencing Skin biopsy and LSGB | TTR gene sequencing DPD scintigraphy | TTR gene sequencing MRI of the brain and spine PiB PET LM biopsy | TTR gene sequencing Vitreous amyloid deposit |
| Natural history | Time to walking with aid, 7 y | Time to walking with aid, 2.6 y | Terminal heart failure Cardiac arrhythmia | ||
| Survival from diagnosis, y | 10 | 4.7 | 2 to 3 | Unk | Unk |
| Survival from onset, y | 12 | 7.3 | 3 to 4 | 9.3 ± 7.1 | Unk |
| Cause of death | Cachexia Sudden death Sepsis | Heart failure Cachexia Secondary infection | Heart failure Sudden death Stroke | Stroke SAH Status epilepticus | |
| . | ATTRv-PN . | ATTRv-CM . | ATTRv-LM or CNS . | ATTRv-O . | |
|---|---|---|---|---|---|
| Epidemiology | 10 000 | Main variant: V122I: 3.4% of African Americans 40 000 gene carriers Other variants in many countries | 82 cases reported (global) | Ubiquitary (dozens of families) | |
| No. of TTR variants | 100 | 24 | 15 | 5 | |
| Main variants | EO V30M | LO V30M, S77Y, E89Q, and I107V | V122I, T60A, I68L, and L111M | D18G, Y69H V30G, L12P, A25T, V30M, G53E, and Y114C | G83A, R34G, Y69H, K35T, and W41L |
| Age at onset, y | 31.9 ± 7.6 | 64.5 ± 6.5 | 74 ± 7 (rare before 50 y) | 44.9 ± 9.1 | 35-56 |
| Male sex, % | 50 | 86 | 80-85 | Unk | Unk |
| Ethnicity | Portuguese Japanese Brazilian Mallorcan Cypriot | Global | V122I: African Americans and Caribbean Other variants: clusters | Various | Various |
| Duration of symptoms at diagnosis (y) | 2 | 2.8 | 1 | Unk | Unk |
| Main clinical manifestations | Symptoms in the feet Autonomic symptoms Unexplained weight loss | Neuropathic (80%) Autonomic (10%) Cardiac (4%-20%) | Heart failure symptoms Fluid overload History of CTS 50% | Cognitive impairment Ataxia Headache Seizures Stroke SAH Hearing loss | Blurred vision Vitreous opacities Glaucoma |
| Penetrance | High 80% at 50 y | High 80% at 80 y | Low | ||
| Tools for diagnosis | TTR gene sequencing Skin biopsy and LSGB | TTR gene sequencing Skin biopsy and LSGB | TTR gene sequencing DPD scintigraphy | TTR gene sequencing MRI of the brain and spine PiB PET LM biopsy | TTR gene sequencing Vitreous amyloid deposit |
| Natural history | Time to walking with aid, 7 y | Time to walking with aid, 2.6 y | Terminal heart failure Cardiac arrhythmia | ||
| Survival from diagnosis, y | 10 | 4.7 | 2 to 3 | Unk | Unk |
| Survival from onset, y | 12 | 7.3 | 3 to 4 | 9.3 ± 7.1 | Unk |
| Cause of death | Cachexia Sudden death Sepsis | Heart failure Cachexia Secondary infection | Heart failure Sudden death Stroke | Stroke SAH Status epilepticus | |
CTS, carpal tunnel syndrome; LM, leptomeningeal biopsy; LSGB, labial salivary gland biopsy; MRI, magnetic resonance imaging; PiB PET, Pittsburgh compound B (PiB)–PET; SAH, subarachnoid hemorrhage; Unk, unknown.
Transthyretin
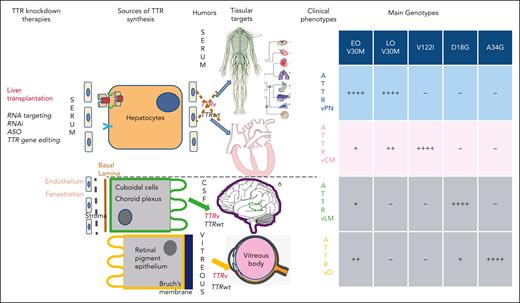
Transthyretin, that is, prealbumin, is a 55 kilodalton homotetrameric protein synthesized predominantly by the liver but also in the choroid plexuses and retinal pigment epithelium.2 Its main function is the transport of thyroxine and retinol-binding protein–vitamin A complex (holoRBP). More than 140 TTR gene variants have been reported until now.8 Predominant local TTR synthesis results in different clinical phenotypes, including (1) neuropathic, cardiac, mixed cardiac, and neuropathic amyloidosis; (2) ocular amyloidosis (vitreous); and (3) leptomeningeal amyloidosis (Figure 1).
Sources of TTR synthesis, humors where ATTRv and ATTRwt are released, tissular target of misfolded TTR, and main clinical phenotypes according to genotypes. V122I:V102I, D18G, and A34G:R34G.
Sources of TTR synthesis, humors where ATTRv and ATTRwt are released, tissular target of misfolded TTR, and main clinical phenotypes according to genotypes. V122I:V102I, D18G, and A34G:R34G.
By convention, TTR amyloid protein is named ATTR.15 Protein denomination can be specified further, for example, ATTRv for ATTR variant and ATTRV30M for the specific TTR gene V30M mutation. wtTTR may cause late onset systemic ATTRv-CM (ATTRwt), mainly involving older males (mean age, 72 years),16 with a median survival of 46 months.17
Phenotypes
ATTRv-PN
ATTRv-PN was first reported in northern Portugal, Japan, and Sweden, associated with the TTRV30M variant. In Portugal and Japan, the disease manifested in the third decade with peripheral polyneuropathy with predominant sensory and autonomic manifestations. Inaugural manifestations include feet paresthesia and pain of insidious onset in association with alternating diarrhea and constipation, erectile dysfunction, and weight loss. Family history is almost constant.18 LO ATTR-V30M (age, >50 years) cases were first reported in Sweden but are now observed in many countries,16 manifesting as a predominant progressive idiopathic sensorimotor polyneuropathy.19 Diagnostic tools include TTR gene sequencing to detect the 140 amyloidogenic TTR variants8 and mini-invasive biopsies to detect amyloid deposits.9 World ATTRv-PN prevalence is estimated to be 10 000.20 Sensory loss progressively expands across the body, autonomic dysfunction worsens, and walking difficulties progressively increase.21 The LO ATTRV30M and other ATTRv-PN variants present with early walking difficulties 2 to 3 years after disease-onset vs 5 years in patients with EO ATTRV30M. Walking difficulties are assessed by the Peripheral Neuropathy Disability (PND) scale (PND 1, no walking difficulties; PND2, walking difficulties unaided; PND2A, walking difficulties requiring 1 aid; PND2B, walking difficulties requiring 2 aids for walking; and PND3, using a wheelchair or bedridden).22 On examination, there is, in parallel, a progressive weakness in grip strength.23 Survival ranges from 7 years in LOV30M and most variants4 to 12 years in EOV30M (Table 1).24
Hereditary TTR ATTRv-CM
Prevalence of ATTRv-CM
It is estimated that 10% to 20%27 of patients with ATTR-CM treated by cardiologists are TTR gene variant carriers.
There are 28 variants associated with ATTRv-CM;8 the main ones being V122I, L111M, T60A, and I68L, according to the international Transthyretin Amyloidosis Outcome Survey (THAOS) registry.16 The most prevalent TTR variant in the United States is V122I, also known as pV142I and V122I. The prevalence of ATTRv-CM ranges from 1 in 100 000 to 1 in 200 000 in the general population. It is estimated to affect about 40 000 people worldwide.25 It is estimated that 3.5% of the African American population, that is, 1.5 million people, carry this specific TTR mutation. In a genome-wide association study performed in the UK Biobank, the cumulative incidence of carpal tunnel syndrome, polyneuropathy, cardiomyopathy, or heart failure was more common in V122I carriers than in noncarriers (hazard ratio [HR], 2.8; 95% confidence interval [CI], 1.7-4.5; P = 2.6 × 10−5), with 37.4% of V122I carriers having at least 1 of these manifestations by age 75 years.28
Interestingly, the population of patients with ATTR-CM is regularly revised upward, probably because ATTRwt prevalence was initially underestimated.
ATTRv-CM clinical manifestations include heart failure, with preserved ejection fraction, and atrial fibrillation. Diagnosis may be suspected using electrocardiogram and/or cardiac echocardiography. Diagnosis confirmation requires TTR gene sequencing and a positive bone scintigraphy, with the cardiac uptake at least as strong as that of bones (Table 1).25
Diagnosis of ATTR-CM and differential diagnosis between ATTR and AL ATTRv-CM
Indeed, even if bisphosphonate scintigraphy is the cornerstone of the ATTR-CM diagnosis, cardiac uptake may also occur in 10% of amyloid light chain (AL) amyloidosis.29 Furthermore, monoclonal gammopathy of uncertain significance increases with age and affects 5% of the patients aged >70 years and, thus, could occur in patients with true ATTR-CM. Finally, some mixed forms with both AL and ATTR deposits have already been described. Therefore, an active search for a monoclonal protein (by serum and urine immunofixation and serum free light chain (FLC) quantification) must be performed as the first diagnostic procedure for all patients. In the presence of the monoclonal protein, tissue biopsy and amyloid typing are mandatory. If there is no monoclonal protein, then the diagnosis of cardiac transthyretin amyloidosis can be performed using a noninvasive pathway with bone scintigraphy only, with no need for a biopsy.
Natural history and staging
The leading cause of death among patients with ATTRv-CM is terminal heart failure.30 Thromboembolic events (stroke and pulmonary embolism) and cardiac arrhythmia (atrial fibrillation, conduction disturbances, ventricular arrhythmia, and electromechanical dissociation) may also occur. Untreated, ATTRv-CM survival after diagnosis ranges between ∼3 and 4 years. In V122I-related ATTRv-CM, survival after diagnosis is 2.7 years.3 However, patients are often diagnosed late. Diagnosis delay can be improved using family segregation genetic analysis, and presymptomatic screening provides a real chance for early diagnosis and management.31 Patients with ATTRv-CM present with progressive heart failure and reduced quality of life.32
Several staging systems have been designed to predict ATTRv-CM survival. Currently, the UK Staging system (or National Amyloidosis Centre (NAC) system) seems the more robust. N-terminal pro-brain natriuretic peptide (NT-proBNP) (≤3000 pg/mL or more) and estimated glomerular filtration rate (eGFR) (≤45 mL/min or more) are the 2 main evaluated parameters. In NAC, stage I is defined by both normal parameters, stage II if 1 parameter is above the threshold, and stage III if both parameters are above threshold. Patients with stage I have a median survival of 69.2 months, decreasing to 46.7 months for stage II and 24.1 months for stage III. A further staging system was published in 2022 to refine prognosis in patients with stage I. For them, a stage Ia was defined as a furosemide equivalent diuretic requirement of <0.75 mg/kg and an NT-proBNP ≤500 ng/L or ≤1000 ng/L in the presence of atrial fibrillation. Stage Ib comprised all remaining patients with stage I.33 Of note, all staging and prognostic tools, including both genotype and phenotype, ultimately retained only phenotypic characteristics in the multivariate models.33-35 This reflects phenotypic variability associated with 1 genotype.
Other phenotypes: leptomeningeal ATTRv and ocular ATTRv
Some patients with non-V30M present with inaugural ocular and/or CNS dysfunction (oculoleptomeningeal amyloidosis), with little systemic involvement. This phenotype is rare and associated with 14 TTR variants associated with CNS leptomeningeal amyloidosis (ATTRv-LM)6 and 5 TTR variants associated with ocular TTR amyloidosis including Gly83Arg7 (Table 1). They are due to predominant TTR synthesis by choroid plexus in cerebrospinal fluid (CSF)6 or by retinal pigment epithelium into vitreous body, respectively7 (Figure 1).
Onset of ATTRv-LM is usually early, and survival is short, ∼9.7 years. Stroke, subarachnoid hemorrhage, and status epilepticus are the main causes of death. Survivors of EO ATTRV30M-PN long LT may also develop CNS symptoms, after at least 14 years of symptomatic peripheral nerve disease.36 ATTRv-LM neurological symptoms include cognitive impairment, headache, ataxia, seizures, and hearing and visual loss. CSF analysis shows increased CSF protein levels, and leptomeningeal enhancement and superficial siderosis may be observed by brain and spine magnetic resonance imaging.6,36 C-Pittsburgh compound B-positron emission tomography (PiB-PET) shows brain amyloid deposition after long-duration ATTRv amyloidosis,37 especially in the cerebellum.38
Main manifestations of ocular amyloidosis are alternating and recurrent blurred vision in both eyes. All patients are at risk of developing blindness. Ocular manifestations are not influenced by LT.30
Therapy
State of the art
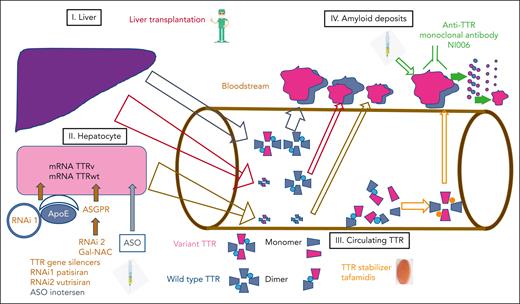
Disease-modifying therapies have emerged in hereditary transthyretin amyloidosis after successive phase 3 clinical trials, resulting in 5 marketing authorizations (Figure 2). They mainly concern ATTRv-PN and include LT, the TTR stabilizers tafamidis and diflunisal, and, more recently, RNA-targeting treatments using silencing RNA and ASO.
Disease-modifying therapies in ATTRv. Targets of disease-modifying therapies are (1) liver, (2) hepatocytes, (3) circulating TTR, and (4) amyloid deposits in organs (heart, kidneys) and peripheral nervous system.
Disease-modifying therapies in ATTRv. Targets of disease-modifying therapies are (1) liver, (2) hepatocytes, (3) circulating TTR, and (4) amyloid deposits in organs (heart, kidneys) and peripheral nervous system.
ATTRv-PN
LT
Therapeutical strategies target hepatocytes, which are the main source of transthyretin released into circulation (Figure 1). The first approach was through LT, which was validated biologically by post-LT, circulating plasma mutant TTRmet30 knockdown39 and clinically by stopping neuropathy aggravation at an early stage.40 LT doubles the survival for patients with the EOV30M variant.11 The limited efficacy of LT in LOV30M and other variants highlights the crucial role of wtTTR in the progression of the disease after LT.41
TTR stabilizers tafamidis and diflunisal
The TTR stabilizer tafamidis is a small molecule binding onto the TTR thyroxine binding site, thus avoiding TTR dissociation into amyloidogenic monomers and dimers. Tafamidis slows progression of the neuropathy in patients with EOV30M42 at early stages but not in overt neuropathy with a high neuropathy impairment score (NIS) > 14.12 In other TTR variants, tafamidis is not able to stop the progression of the disease and disability.43,44
Diflunisal, a nonsteroidal antiinflammatory agent, was another TTR stabilizer that reduced the rate of progression in neurologic impairment and preserved quality of life in a 2-year phase 3 trial in ATTRv-PN of various genotype and severity14 but had no marketing authorization.
RNA targeting
The innovative therapeutical strategy of RNA targeting (Figure 3) emerged in the past 15 years, offering the opportunity of knocking down the production of both variant TTR and wtTTR in hepatocytes using messenger RNA (mRNA) degradation.
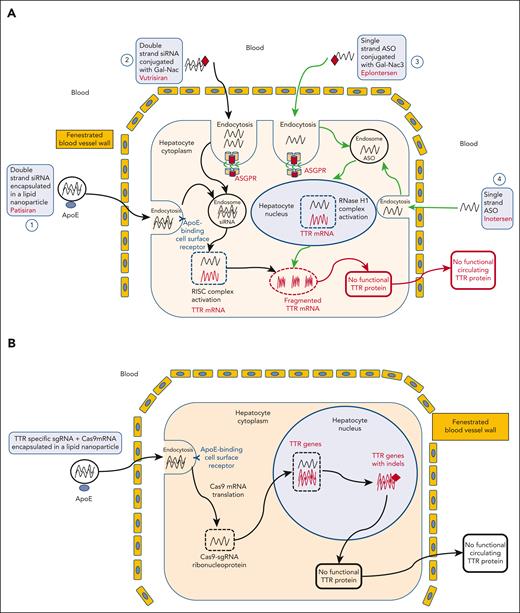
Targeting RNA in hepatocytes. (A) After IV administration, double strand siRNA encapsulated in a lipid nanoparticle, that is, patisiran and is opsonized by apoliprotein E (ApoE), goes to the liver through vascular fenestrations, and binds to ApoE receptors on hepatocytes surface. The antisense strand of the siRNA then enters an enzyme complex called the RNA–inducing silencing complex. The siRNA antisense strand binds to the same sequence within the 3′ untranslated region of both mutant and wtTTR mRNAs, and this enzyme complex subsequently binds to its complementary mRNA target sequence, mediating its cleavage by argonaute-2 endonuclease (Ago2), thereby preventing synthesis of the target protein ATTRv and ATTRwt (pathway 1). Another approach uses a siRNA conjugated to a trimer of Gal-NAC residues, that is, vutrisiran, which is recognized by and transported into hepatocytes by asialoglycoprotein receptors (ASGPRs) located on the surface of hepatocytes. After the ligand-receptor complex is internalized, the cargo is released into the endocytic pathway with subsequent engagement with the RNA-induced silencing complex (RISC). ASGPR is then rapidly recycled to the cell surface, thus enabling multiple rounds of cargo uptake and release (pathway 2). Second-generation ASO inotersen selectively targets liver TTR mRNA, hybridizing to the 30-nontranslated regions that contain no known TTR mutations. Inotersen binds to wt and mutant transthyretin RNA transcripts, resulting in their degradation in the nucleus by ribonuclease H. As a result, inotersen inhibits the production of wtTTR and all mutant forms of TTR (pathway 4). Eplontersen is conjugated to a triantennary GalNAc3 moiety to facilitate delivery to hepatocytes through ASGPR-mediated uptake (pathway 3). (B) TTR gene editing a TTR-specific single guide RNA (sgRNA) and a Cas9 mRNA encapsulated in a lipid nanoparticle is opsonized by ApoE, goes to the liver through vascular fenestrations, and binds to ApoE receptors at hepatocytes surface. Cas 9 mRNA is then translated in the cytoplasm, and the Cas9-sgRNA ribonucleoprotein enters the nucleus and provokes targeted DNA cleavage. As a result, endogenous DNA repair induces the appearance of indels in the TTR gene, leading to frameshift mutations impairing functional TTR protein production.
Targeting RNA in hepatocytes. (A) After IV administration, double strand siRNA encapsulated in a lipid nanoparticle, that is, patisiran and is opsonized by apoliprotein E (ApoE), goes to the liver through vascular fenestrations, and binds to ApoE receptors on hepatocytes surface. The antisense strand of the siRNA then enters an enzyme complex called the RNA–inducing silencing complex. The siRNA antisense strand binds to the same sequence within the 3′ untranslated region of both mutant and wtTTR mRNAs, and this enzyme complex subsequently binds to its complementary mRNA target sequence, mediating its cleavage by argonaute-2 endonuclease (Ago2), thereby preventing synthesis of the target protein ATTRv and ATTRwt (pathway 1). Another approach uses a siRNA conjugated to a trimer of Gal-NAC residues, that is, vutrisiran, which is recognized by and transported into hepatocytes by asialoglycoprotein receptors (ASGPRs) located on the surface of hepatocytes. After the ligand-receptor complex is internalized, the cargo is released into the endocytic pathway with subsequent engagement with the RNA-induced silencing complex (RISC). ASGPR is then rapidly recycled to the cell surface, thus enabling multiple rounds of cargo uptake and release (pathway 2). Second-generation ASO inotersen selectively targets liver TTR mRNA, hybridizing to the 30-nontranslated regions that contain no known TTR mutations. Inotersen binds to wt and mutant transthyretin RNA transcripts, resulting in their degradation in the nucleus by ribonuclease H. As a result, inotersen inhibits the production of wtTTR and all mutant forms of TTR (pathway 4). Eplontersen is conjugated to a triantennary GalNAc3 moiety to facilitate delivery to hepatocytes through ASGPR-mediated uptake (pathway 3). (B) TTR gene editing a TTR-specific single guide RNA (sgRNA) and a Cas9 mRNA encapsulated in a lipid nanoparticle is opsonized by ApoE, goes to the liver through vascular fenestrations, and binds to ApoE receptors at hepatocytes surface. Cas 9 mRNA is then translated in the cytoplasm, and the Cas9-sgRNA ribonucleoprotein enters the nucleus and provokes targeted DNA cleavage. As a result, endogenous DNA repair induces the appearance of indels in the TTR gene, leading to frameshift mutations impairing functional TTR protein production.
RNA interference is a naturally occurring cellular mechanism for regulating gene expression by which a double-stranded short interfering RNA (siRNA) mediates the sequence-specific degradation of mRNA, leading to a reduced synthesis of the corresponding protein. ALN-TTR01 and -TTR02 are first- and second-generation lipid nanoparticle formulations of the same siRNA targeting both variant and wtTTR mRNA, resulting in their degradation by the cytoplasmic dicer small interfering RNA mechanism.45,46 Targeting hepatocytes for siRNA with lipid nanoparticules allows for the adsorption in the hepatocytes by apoliprotein E receptor after IV infusion and requires IV dosing every 3 weeks. Targeting the liver was facilitated by fenestrated hepatic endothelium (Figure 3A).
Inotersen is a single strand 20 nucleotide-long ASO chemically modified with phosphorothioate in the backbone that selectively targets TTR mRNA in the liver. This chimeric ASO is complementary, for hybridizing, to the 30-untranslated region of the human transthyretin mRNA, which contains no known TTR mutations. As a result, inotersen is projected to inhibit the production of wtTTR and all mutant forms of TTR. It was selected for weekly once subcutaneous (SC) dosing regimen in clinical trials for patients with ATTR amyloidosis.47
RNA therapy phase 3 trials against placebo
Two major clinical trials compared RNA therapy RNAi patisiran (APOLLO)46 and ASO inotersen (NEURO-TTR)48 with placebo for patients with ATTRv-PN with various TTR variants and stages of severity (Table 2)
Phase 3 clinical trials APOLLO A with patisiran and NEURO-TTR with inotersen for ATTRv-PN
| . | APOLLO-A . | NEURO-TTR . |
|---|---|---|
| Criteria of inclusion | NIS 5-130 TTR variant 18 to 85 y | NIS 10-130 TTR variant 18 to 82 y Amyloid deposit |
| Class | RNAi | ASO |
| IMP | Patisiran | Inotersen |
| Method of administration | IV∗ | SC |
| Dose | 0.3 mg/kg | 300 mg |
| Rhythm of administration | 1 per 3 wk | 1 per wk |
| Ratio of IMP-to-PB | 2:1 | 2:1 |
| N | 225 | 172 |
| Mean age, y | 59 | 59.2 |
| TTR variants, N | 39 | 27 |
| Val30Met TTR variants, % | 43 | 52 |
| Coutinho stage† 2 to 3 (%) | 54 | 43 |
| mNIS + 7 | 81 | 79.2 |
| Norfolk-QoL-DN | 55.5 | 48.4 |
| Primary end points | CFB of mNIS + 7 at 18 mo | CFB mNIS +7 and QOL-DN at week 66 |
| Secondary end points | CFB Norfolk QOL-DN, 10M-WT, R-ODS, and compass 31 mBMI | CFB Norfolk QOL-DN symptoms domain score, mBMI, and TTR |
| Exploratory end points | TTR, Echocardiography and measurement of NT-proBNP | |
| Results | ||
| Primary end points | ||
| LS mean changes | mNIS + 7 | mNIS + 7 |
| Placebo | +28 | +25.5 |
| IMP | −6.7 | +5.8 |
| % improvement/baseline | ||
| Placebo | 4% | 19% |
| IMP | 56% | 36% |
| Secondary end points | QOL-DN | QOL-DN (Iary end point) |
| Placebo | +19.8 | +12.7 |
| IMP | −6.7 | +1 |
| 10MWT m/s LS mean changes, PL/IMP | −0.24/+0.08 | NA |
| R-ODS LS mean changes, PL/IMP | −8.9/0.0 | NA |
| Compass31 LS mean changes, PL/IMP | +2.2/–5.3 | NA |
| mBMI LS mean changes, PL/IMP | −119/–3.7 | −0.8/–0.3 |
| Exploratory end points | ||
| Serum TTR median reduction level during the 18 mo | 81% | 74% (mean, nadir) |
| Time to reach study state, wk | 3 | 13 |
| Main AEs | Infusion-related reaction Peripheral edema | Thrombocytopenia Glomerulonephritis |
| Completed the intervention period (IMP) | 93% | 78% |
| MA | EMA-FDA | EMA-FDA |
| Coutinho stage 1 and 2 | Coutinho stage 1 and 2 |
| . | APOLLO-A . | NEURO-TTR . |
|---|---|---|
| Criteria of inclusion | NIS 5-130 TTR variant 18 to 85 y | NIS 10-130 TTR variant 18 to 82 y Amyloid deposit |
| Class | RNAi | ASO |
| IMP | Patisiran | Inotersen |
| Method of administration | IV∗ | SC |
| Dose | 0.3 mg/kg | 300 mg |
| Rhythm of administration | 1 per 3 wk | 1 per wk |
| Ratio of IMP-to-PB | 2:1 | 2:1 |
| N | 225 | 172 |
| Mean age, y | 59 | 59.2 |
| TTR variants, N | 39 | 27 |
| Val30Met TTR variants, % | 43 | 52 |
| Coutinho stage† 2 to 3 (%) | 54 | 43 |
| mNIS + 7 | 81 | 79.2 |
| Norfolk-QoL-DN | 55.5 | 48.4 |
| Primary end points | CFB of mNIS + 7 at 18 mo | CFB mNIS +7 and QOL-DN at week 66 |
| Secondary end points | CFB Norfolk QOL-DN, 10M-WT, R-ODS, and compass 31 mBMI | CFB Norfolk QOL-DN symptoms domain score, mBMI, and TTR |
| Exploratory end points | TTR, Echocardiography and measurement of NT-proBNP | |
| Results | ||
| Primary end points | ||
| LS mean changes | mNIS + 7 | mNIS + 7 |
| Placebo | +28 | +25.5 |
| IMP | −6.7 | +5.8 |
| % improvement/baseline | ||
| Placebo | 4% | 19% |
| IMP | 56% | 36% |
| Secondary end points | QOL-DN | QOL-DN (Iary end point) |
| Placebo | +19.8 | +12.7 |
| IMP | −6.7 | +1 |
| 10MWT m/s LS mean changes, PL/IMP | −0.24/+0.08 | NA |
| R-ODS LS mean changes, PL/IMP | −8.9/0.0 | NA |
| Compass31 LS mean changes, PL/IMP | +2.2/–5.3 | NA |
| mBMI LS mean changes, PL/IMP | −119/–3.7 | −0.8/–0.3 |
| Exploratory end points | ||
| Serum TTR median reduction level during the 18 mo | 81% | 74% (mean, nadir) |
| Time to reach study state, wk | 3 | 13 |
| Main AEs | Infusion-related reaction Peripheral edema | Thrombocytopenia Glomerulonephritis |
| Completed the intervention period (IMP) | 93% | 78% |
| MA | EMA-FDA | EMA-FDA |
| Coutinho stage 1 and 2 | Coutinho stage 1 and 2 |
AE, adverse event; CFB, change from baseline; EMA, European medicines agency; FDA, food and drug administration; IMP, investigational medicinal product; LS, least-squares; MA, marketing authorization; mBMI, modified body mass index; NIS, neuropathic impairment score; PB, placebo.
Greater or equal or a 0-point increase from baseline.
Coutinho stage 2 to 3, walking with aid or using a wheelchair.
In comparing these 2 studies point by point, both addressed the investigational medicinal product–to–placebo 2:1 ratio, ATTRv-PN with several TTR variants with a large range of severity, including either Coutinho stage I (walking unaided) or stage II (walking with aid), and NIS at baseline ranging from 5 to 130 for APOLLO A46 and from 10 to 130 for NEURO-TTR. NEURO-TTR required documented amyloid deposits determined via biopsy for inclusion. Duration of the trial was 18 months for RNAi and 66 weeks for ASO. In a phase 3 trial APOLLO-A, 225 patients with ATTRv-PN were randomly assigned to receive IV patisiran (0.3 mg/kg) or placebo once every 3 weeks.46 A total of 172 patients were randomly assigned to receive weekly SC injections of inotersen (300 mg) or placebo.
Concerning the design, there was 1 primary end point change for modified NIS + 7 (mNIS + 7) from baseline for APOLLO-A and 2 coprimary end points change of the composite mNIS + 749 and Norfolk-auality of life (QOL) from baseline for NEURO-TTR. APOLLO trial included several secondary end points to assess the clinically pertinent impact on daily life: walking speed, disability Rasch-built Overall Disability Scale, and autonomic dysfunction by COMPASS31 score questionnaire, conversely to NEURO-TTR. The level of serum TTR knockdown was assessed in both trials.
Concerning the results, in both studies, LS mean changes of mNIS + 7 increased in placebo groups during the trial by 28 or 25.5 points, with minor increase in ASO group by +5.8, but a decrease by 6 points, meaning improvement in the patisiran group. Up to 56% of patients receiving patisiran improved mNIS + 7 compared with baseline vs 4% in placebo, vs 36% with inotersen, and vs 19% with placebo, knowing that the definition of improvement was different in the studies: no increase from the baseline for NEURO-TTR and only decrease from baseline in APOLLO-A.
Norfolk-QOL increased in both trials in the placebo group, with a minor increase by +1% in NEURO-TTR but a decrease by −6.7 in APOLLO-A trial with patisiran, meaning improvement of QOL.
Interestingly, patisiran allowed for increased walking speed, improved autonomic function, and slowed disability progression.
Serum TTR decrease was faster with patisiran vs inotersen, that is, 3 weeks vs 13 weeks. Patisiran had a good safety profile, with only infusion-related reactions and peripheral edema in a few cases.46 When considering inotersen, 3 patients developed severe thrombocytopenia, including 1 fatal cerebral hemorrhage, and 3 patients developed glomerulonephritis.48
In an observational retrospective study of 23 patients with ATTRv-PN treated with inotersen, 52% discontinued treatment or reduced dosing frequency because of recurrent thrombocytopenia.50
Clinical trials with RNAi–Gal-NAC vs patisiran
N-acetyl galactosamine (Gal-NAC) ligand allows for SC injection and liver-addressing using ASPGR receptors.51,52 Gal-NAC siRNA modification allows for a prolonged intrahepatocellular action through trapped endosomal siRNA, slow cytoplasmic release, and prolonged RNAi response, thus allowing for mRNA and TTR knockdown more than 3 months after dosing53,54 and quarterly SC injections52,55 (Figure 3A).
Helios-A study evaluated the efficacy and safety of Gal-NAC siRNA vutrisiran for patients with ATTRv-PN.55 Participants received vutrisiran or patisiran during the treatment period at a 3:1 ratio. The placebo arm of the APOLLO study was used as an external comparator, and vutrisiran met the primary end point change from the baseline in the mNIS + 7 arm at month 9 and then all secondary efficacy end points. TTR reduction with vutrisiran was similar to patisiran over 18 months. Five patients (4.1%) who received vutrisiran reported mild injection-site reactions.
OLE studies with RNA targeting
Open-label extension (OLE) studies confirmed the biological impact and benefit of RNA targeting in the course of the disease.56,57 However, delayed treatment introduction of patisiran has a negative impact on survival.56 After 36 months, half of patients enrolled in inotersen OLE trial were responders defined by a change in the mNIS + 7 score of <12.2 points from baseline. Platelet and renal monitoring was effective in preventing significant adverse events.57
RNAi therapy was also proposed in 23 patients with ATTRv-PN progression after LT in a phase 3b, open-label trial evaluating the efficacy and safety of patisiran58 who received patisiran alongside immunosuppression regimens. Patisiran allowed for a median TTR reduction of 91.0%, improved neuropathy, QOL, and autonomic symptoms from baseline to month 12, and stabilized Rasch-built Overall Disability Scale. Nine patients with post-LT ATTRv were treated with inotersen in the extended access program for a median time of 12 months, with a stable NIS. Five (56%) patients stopped treatment because of thrombocytopenia or reversible liver rejection.59
ATTRv-CM
Today, the only disease-modifying therapy approved in ATTRv-CM is tafamidis, a TTR stabilizer. RNAi therapy has been tested in ATTRv-CM, with a first line siRNA revusiran, and later with 2 compounds (patisiran and vutrisiran). Several innovative treatment approaches are under clinical development in ATTRv-CM.
TTR stabilizer
In the ATTR-ACT trial, tafamidis increased survival in ATTRv-CM by 13% compared with placebo at 30 months.13 As of 2023, tafamidis is the only medication approved to treat ATTR-CM, both in the United States and Europe.60 Published in 2018, this phase 3 trial compared tafamidis (20 mg and 80 mg) with placebo for 441 patients with ATTR-CM, including 106 patients with ATTRv (24%) and 335 patients with ATTRwt (76%). At month 30, up to 57.1% of the patients treated with placebo were alive vs 70.5% of patients treated with tafamidis. Prespecified subgroup analysis showed consistent benefits according to genotype (ATTRv vs ATTRwt) and New York Heart Association classes (1 and 2 vs 3). It seemed that patients who benefited the most from tafamidis were those with the earliest diagnosis and the lightest stage.
RNAi therapy for ATTRv-CM
The use of RNA silencers to treat ATTRv-CM came close to being abandoned. Indeed, the first siRNA phase 3 trial was conducted with revusiran (ENDEAVOUR) (Table 3).61 After having included 140 patients treated with revusiran and 66 treated with placebo, the trial was terminated prematurely because of excess mortality in the treatment group (revusiran vs placebo, 18 vs 2). No clear causative mechanism could be evidenced. It was noteworthy that revusiran was a first-generation siRNA conjugated with Gal-NAC that required SC injection of high doses to reach the effects on TTR (500 mg injected each week vs 0.3 mg/kg every 3 weeks for patisiran and 25mg every 3 months for vutrisiran). This raises the question of off-target effects with high doses of siRNA.
Phase 3 clinical trials with RNAi therapy siRNA and ASO in ATTRv-CM
| Study . | ENDEAVOUR . | APOLLO-B . | HELIOS B . | CARDIO-TTRANSFORM . |
|---|---|---|---|---|
| Main criteria of inclusion | ATTRv-CM, NYHA∗ < 4 PND†< 3, and TTR mutation Amyloid deposits MH of heart failure Involvement by echocardiogram | ATTRv-CM or ATTRwt-CM MH of heart failure | ATTRv-CM or ATTRwt-CM MH of heart failure | ATTRv-CM or ATTRwt-CM Interventricular septum thickness > 12 mm NYHA 1 to 3 |
| IMP | Revusiran | Patisiran | Vutrisiran | Eplontersen |
| Method of administration | SC | IV | SC | SC |
| Dose | 500 mg | 0.3 mg/kg | 25 mg | 45 mg |
| Rhythm of administration | 1 daily for 1 wk Then 1 every wk | Every 3 wk | Every 3 mo | Every month |
| N | 206 | 360 | 655 | 1400 |
| ATTRv, % | 100 | 20 | Unk | Unk |
| Variants | V122I, 57%; T60A, 16% | Unk | ||
| Age, y | 69 | 76 | Unk | Unk |
| NYHA3, stage, % | 31 | 8 | 0 (exclusion criteria) | Unk |
| Primary end points | CFB in 6-MWT to 18 mo; % reduction in serum TTR levels over 18 mo | CFB at mo 12 in 6-MWT | Composite end point of all-cause mortality and recurrent CV events | Composite outcome of CV mortality and recurrent CV clinical events up to wk 140 |
| End of trial | March 2017 | June 2022 | June 2024 | June 2025 |
| Results | Revusiran treatment was stopped after a median of 6.71 mo | Not yet | Not yet | |
| Primary end point | Not reached | Positive results on primary end point 6-MWT patisiran vs placebo: −8.15 mo vs −21.35 mo; P = .0162. At 12 mo∗ No effects on survival. | ||
| Safety | Mortality imbalance between treatment arms: 18 patients (12.9%) on revusiran and 2 (3.0%) on placebo during the on-treatment period | |||
| TTR knockdown | Mean > 80% reduction of serum TTR, mo 1 to 15 | Mean % reduction from baseline in serum TTR of 87% at mo 12 | ||
| Marketing authorization | No | No (not yet) | No | No |
| Study . | ENDEAVOUR . | APOLLO-B . | HELIOS B . | CARDIO-TTRANSFORM . |
|---|---|---|---|---|
| Main criteria of inclusion | ATTRv-CM, NYHA∗ < 4 PND†< 3, and TTR mutation Amyloid deposits MH of heart failure Involvement by echocardiogram | ATTRv-CM or ATTRwt-CM MH of heart failure | ATTRv-CM or ATTRwt-CM MH of heart failure | ATTRv-CM or ATTRwt-CM Interventricular septum thickness > 12 mm NYHA 1 to 3 |
| IMP | Revusiran | Patisiran | Vutrisiran | Eplontersen |
| Method of administration | SC | IV | SC | SC |
| Dose | 500 mg | 0.3 mg/kg | 25 mg | 45 mg |
| Rhythm of administration | 1 daily for 1 wk Then 1 every wk | Every 3 wk | Every 3 mo | Every month |
| N | 206 | 360 | 655 | 1400 |
| ATTRv, % | 100 | 20 | Unk | Unk |
| Variants | V122I, 57%; T60A, 16% | Unk | ||
| Age, y | 69 | 76 | Unk | Unk |
| NYHA3, stage, % | 31 | 8 | 0 (exclusion criteria) | Unk |
| Primary end points | CFB in 6-MWT to 18 mo; % reduction in serum TTR levels over 18 mo | CFB at mo 12 in 6-MWT | Composite end point of all-cause mortality and recurrent CV events | Composite outcome of CV mortality and recurrent CV clinical events up to wk 140 |
| End of trial | March 2017 | June 2022 | June 2024 | June 2025 |
| Results | Revusiran treatment was stopped after a median of 6.71 mo | Not yet | Not yet | |
| Primary end point | Not reached | Positive results on primary end point 6-MWT patisiran vs placebo: −8.15 mo vs −21.35 mo; P = .0162. At 12 mo∗ No effects on survival. | ||
| Safety | Mortality imbalance between treatment arms: 18 patients (12.9%) on revusiran and 2 (3.0%) on placebo during the on-treatment period | |||
| TTR knockdown | Mean > 80% reduction of serum TTR, mo 1 to 15 | Mean % reduction from baseline in serum TTR of 87% at mo 12 | ||
| Marketing authorization | No | No (not yet) | No | No |
6-MWT, 6-minute walk test, CFB, change from baseline; CV, cardiovascular; IMP, Investigational medicinal product; KCCQ, The Kansas City Cardiomyopathy Questionnaire; MH, medical history; NYHA, New York Heart Association; Unk, unknown.
Results have been presented but are not published yet.
CV hospitalizations and urgent heart failure visits.
The APOLLO-A study was also relevant from a cardiac point of view. Indeed, half of the patients included in this trial presented a mixed phenotype, with both ATTR-PN and ATTR-CM. Analysis of the secondary exploratory criteria (echocardiography biomarkers) suggested a stabilization of the cardiac disease.62,63
Thus, these results led to the completion of the APOLLO-B study, which was presented in September 2022 (NCT03997383). This phase 3 trial included 360 patients (randomization in 1:1 for patisiran vs placebo). The primary end point (6-minute walk test at 12 months) was less altered with patisiran than with placebo. Analyses of the secondary end points under patisiran showed quality of life (The Kansas City Cardiomyopathy Questionnaire) stabilization and a less marked increase of heart failure markers (NT-proBNP and echocardiographic parameters). As expected in view of the number and duration of follow-up, the survival analysis did not show any significant difference between the 2 groups. The safety analysis was good, with no excess mortality. OLE has begun, and publication is awaited (NCT05505838).
Innovative treatment approaches under clinical development
Several innovative treatments are under clinical development; they include new TTR stabilizer, Gal-NAC-siRNA, Gal-NAC ASO, anti-TTR immunotherapy, and TTR gene editing. Each trial will include both ATTRwt-CM and ATTRv-CM.
TTR stabilizer acoramidis
AG10 (acoramidis) is a small-molecule TTR stabilizer highly selective, with a good overall tolerability and safety after sustained oral dosing in a phase 2 study.64 TTR stability was assessed in 2 established ex vivo assays for patients with symptomatic ATTR-CM.61,65 After having failed to reach the 12-month end point (6-minute walk test), the ATTRibute-CM trial will evaluate a hierarchical combination of all-cause mortality and frequency of cardiovascular-related hospitalization, measured at month 30 (NCT03860935).65
Vutrisiran for ATTR-CM
HELIOS B is a large phase 3 trial initiated to test vs placebo the efficacy (and the safety) of vutrisiran to reduce morbimortality in ATTR-CM (NCT04153149). HELIOS B has reached its enrollment target (655 patients). The primary end point is composite and includes all-cause mortality and recurrent cardiovascular (CV) events evaluated at 36 months after inclusion. Results are awaited in 2024.
Eplontersen
Eplontersen is a triantennary Gal-NAC–conjugated ASO allowing for liver-targeting.66,67 Eplontersen 45 mg SC once every 4 weeks produced sustained decreases in serum TTR levels within 30 days of treatment initiation, achieving a mean reduction of 86% after 4 doses of treatment68 (Figure 3A). NEUROTTRansform is a phase 3 multicenter, open-label, randomized study of patients with ATTRv-PN assigned to receive monthly eplontersen or an active reference arm inotersen. The primary aim is to evaluate the efficacy of eplontersen for patients with ATTRv-PN relative to that of the historical placebo control arm of the NEURO-TTR trial. Better safety and equal efficacy are hoped for eplontersen.69 Launched in 2020, the phase 3 trial CARDIO-TTRansform, to our knowledge, is currently the largest phase 3 trial ongoing for ATTR-CM (NCT04136171). The study will include 1400 patients with ATTR-CM. Patients will be randomized between eplontersen and placebo. The primary end point is composite and includes CV mortality and recurrent CV clinical events up to week 140. Results are expected in 2025.
TTR genome editing CRISPR-Cas9 therapy
The hope of a 1-time treatment definitively silencing TTR production using CRISPR-Cas9 technology is emerging. This approach requires specific hepatic targeting, nucleus entry, and TTR gene specific alteration. That has been achieved by encapsulating mRNA for Cas9 protein and a single guide RNA targeting TTR in lipid nanoparticles (Figure 3B).70 In vitro, in transgenic mice and primates, 94% TTR reduction was obtained over a period of 12 months.70 In the ongoing NTLA-2001 phase 1 trial, 6 patients with ATTRv-PN were administered a single IV CRISPR-Cas9 dose. On day 28, mean serum TTR protein was reduced by 87% (range, 80% to 96%) in the 0.3 mg/kg group. Adverse events were rare and mild.70 However, long-term follow-up is needed for detecting potential off-target effects.
Anti-TTR immunotherapy
The aim of monoclonal antibody (Mab)-TTR is the clearance of amyloid deposit. NI301A, a Mab-TTR, removes ATTR deposits ex vivo from patient-derived myocardium by macrophages as well as in vivo from mice grafted with patient-derived ATTR fibrils (amyloid).71 Finally, the recombinant human anti-ATTR antibody NI006 has been recently tested in a phase 1 double blind trial for 40 patients72 (Figure 2). The drug was well tolerated. There was a trend toward a reduction of the cardiac tracer uptake on bone scintigraphy and toward the extracellular volume on cardiac magnetic resonance (CMR) imaging. A phase 3 trial will soon be initiated.
Limits and future of RNA targeting
RNA-targeting trials for patients with ATTRv-CM are in progress. However, small RNA does not go through the blood-brain barrier, and, as a consequence, choroidal cells and retinal pigment epithelium73 involved in ATTRv leptomeningeal and ocular amyloidosis are not targeted. Preclinical attempts to target choroid plexus by intraventricular ASO allows for a 50% CSF TTR reduction in transgenic TTR.74 There was a failure in RNAi to access TTR released by CSF.75
Current strategies for treating patients with ATTRv
Guidelines for ATTRv amyloidosis therapy include drugs with marketing authorization. Patients with early-stage neuropathy should be treated with either tafamidis, patisiran, or inotersen. Patients with mixed cardiac and neurological phenotype should be treated with tafamidis because tafamidis is the only drug with a marketing authorization for cardiomyopathy. Cardiomyopathy may also be treated with gene silencer on the basis of indirect benefit evidence.76 It is now known that delaying RNA therapy has negative impact on survival.56
Conclusions
Hepatic RNA targeting in hereditary ATTR amyloidosis transformed the ATTRv-PN story, and it is hoped that it will be the same with ATTRv-CM. To tame ATTRv, targeting the choroid plexus and retina is now paramount.
Authorship
Contribution: All authors drafted and reviewed the manuscript and approved its final version.
Conflict-of-interest disclosure: D.A. received consulting fees from Alnylam, Pfizer, and AstraZeneca. V.A. received consulting fees and research grants from Alnylam and Pfizer. A.E.-L. received consulting fees from Alnylam Pharmaceuticals, Akcea Therapeutics, and Pfizer.
Correspondence: David Adams, Service de Neurologie, CHU Bicêtre, Assistance Publique–Hôpitaux de Paris, University of Paris Saclay, 78 rue du Général Leclerc, Le Kremlin Bicêtre, 94270 Paris, France; e-mail: david.adams@aphp.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal