Key Points
Patients with IGHV-M have favorable very-long–term PFS after FCR, although later relapses (>10 years) can occur, albeit rarely.
Cumulative risk of tMNs in all patients was 6.3%; patients with IGHV-M are more likely to die from causes other than CLL.
Abstract
Chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab (FCR) achieves durable remissions, with flattening of the progression-free survival (PFS) curve in patients with mutated immunoglobulin heavy chain variable gene (IGHV-M). We updated long-term follow-up results from the original 300-patient FCR study initiated at MD Anderson in 1999. The current median follow-up is 19.0 years. With this extended follow-up, the median PFS for patients with IGHV-M was 14.6 years vs 4.2 years for patients with unmutated IGHV (IGHV-UM). Disease progression beyond 10 years was uncommon. In total, 16 of 94 (17%) patients in remission at 10 years subsequently progressed with the additional follow-up compared with the patients in our prior report in 2015. Only 4 of 45 patients (9%) with IGHV-M progressed beyond 10 years. Excluding Richter transformation, 96 of 300 patients (32%) developed 106 other malignancies, with 19 of 300 (6.3%) developing therapy-related myeloid neoplasms (tMNs), which were fatal in 16 of 19 (84%). No pretreatment patient characteristics predicted the risk of tMNs. In summary, FCR remains an option for patients with IGHV-M chronic lymphocytic leukemia (CLL), with a significant fraction achieving functional cure of CLL. A risk-benefit assessment is warranted when counseling patients, balancing potential functional cure with the risk of late relapses and serious secondary malignancies.
Introduction
We1 and others2,3 previously reported long-term progression-free survival (PFS) after first-line fludarabine, cyclophosphamide, and rituximab (FCR) treatment for chronic lymphocytic leukemia (CLL), raising the possibility of functional cure in a significant fraction of patients with mutated immunoglobulin heavy chain variable gene (IGHV-M). However, there is concern regarding a 2% to 8% risk of therapy-related myeloid neoplasms (tMNs) after FC or FCR administration.1,3-6
First-line ibrutinib with or without ritumixab (IR) showed superior PFS compared with chemoimmunotherapy7,8 and in the E1912 study, superior overall survival (OS).7 The recently updated E1912 results showed a PFS benefit for the IR arm in the patients with IGHV-M at a median follow-up of 6 years.9 However, the survival difference seen in the E1912 study was not seen in the FLAIR trial of FCR vs IR,10 and the PFS benefit requires indefinite ibrutinib therapy, which is associated with high cost11 and a continuous risk of adverse effects, including sudden cardiac death.10 First-line venetoclax plus obinutuzumab treatment in the CLL13/GAIA study was associated with higher rates of undetectable measurable residual disease (MRD) than FCR treatment, with only 1 year of therapy.12 However, with a current median follow-up time of 38.8 months, there is no PFS difference between venetoclax-based regimens and chemoimmunotherapy (3-year PFS, 87%-96% vs 89.9% in patients with IGHV-M).13 Currently, excluding allogeneic stem cell transplantation, FCR remains the only treatment known to produce long-term, functional cure in patients with IGHV-M; it is, therefore, critical to understand the natural history of this patient cohort.
Study design
The study design was reported previously.1,14,15 Many patients returned to their referring centers for long-term follow-up, but relapse and survival data continued to be collected (supplemental Methods, available on the Blood website). All patients gave informed consent for participation, and the study was conducted according to the Declaration of Helsinki.
Statistical considerations
Progressive disease was defined according to the International Working Group in Chronic Lymphocytic Leukemia 1996 criteria. PFS was defined as the time from initiation of therapy until the development of progressive disease, initiation of salvage therapy, or death. In total, 14 patients (5%) were censored for PFS when they received systemic therapy for another cancer while remaining in clinical remission for CLL, but continued to be followed up for OS. Survival was calculated using the Kaplan-Meier method, and comparisons were made using the log-rank test; hazard ratios (HRs), including 95% confidence intervals (CIs) were calculated using Cox regression. Median follow-up time was calculated using the reverse Kaplan-Meier method.
We computed the expected survival for a reference population matched on age, sex, and year using the most recent life tables for the US population from the Human Mortality Database.16 We used a 1-sample log-rank test to compare survival for cohorts based on IGHV mutation status (IGHV-MS) to the expected survival for a matched population.
Prognostic factor evaluation
IGHV-MS and MRD (by ligase chain reaction) status were determined as described previously.1 Fluorescent in situ hybridization analysis for del(11q) and del(17p) was not performed at MD Anderson Cancer Center at the time of enrollment of the study.
Results and discussion
Pretreatment characteristics of patients (n = 300) are shown in supplemental Table 1. IGHV-MS was mutated in 88 patients, unmutated (IGHV-UM) in 126 patients, and unknown in 86 patients, mainly because of lack of available samples.
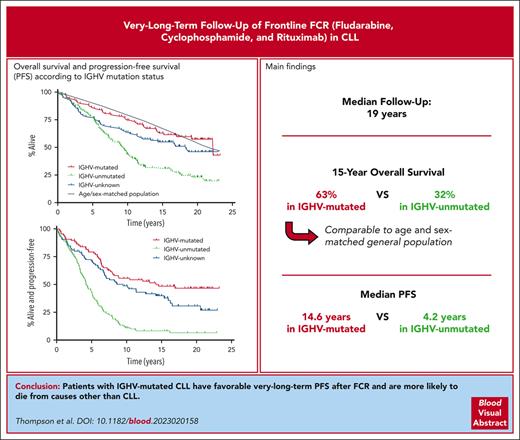
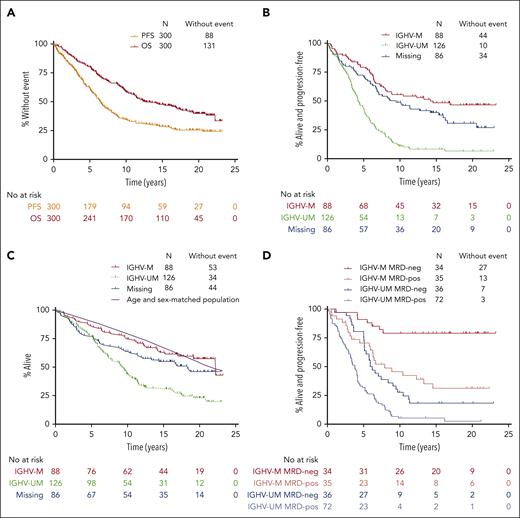
The median follow-up time was 19.0 years. The median PFS was 6.4 years for the whole population (Figure 1A). Median PFS was 14.6 years in patients with IGHV-M and 4.2 years in patients with IGHV-UM (HR for progression or death, 3.5; 95% CI, 2.4-5.0; P < .001). At 15 years of follow-up, 8.3% of patients at risk with IGHV-UM remained alive and progression-free vs 48.7% of patients with IGHV-M (Figure 1B). Among 94 patients in remission for >10 years, 16 progressed. Four of 45 patients with IGHV-M who were in remission at 10 years subsequently progressed, and 2 died in remission: 1 from chronic obstructive pulmonary disease and 1 from unknown causes.
Prognostic factor analysis for long-term PFS and OS for the FCR300 cohort. (A) PFS and OS for the overall cohort. (B) PFS according to IGHV-MS. (C) OS according to IGHV-MS. The purple line represents expected survival of a US reference population matched for age, sex, and year. (D) PFS according to IGHV and MRD status at the end of therapy. Neg, undetectable MRD; pos, MRD-positive.
Prognostic factor analysis for long-term PFS and OS for the FCR300 cohort. (A) PFS and OS for the overall cohort. (B) PFS according to IGHV-MS. (C) OS according to IGHV-MS. The purple line represents expected survival of a US reference population matched for age, sex, and year. (D) PFS according to IGHV and MRD status at the end of therapy. Neg, undetectable MRD; pos, MRD-positive.
The median OS was 12.7 years for the whole population (Figure 1A). The 15-year OS was 63.1% in patients with IGHV-M and 32.0% in patients with IGHV-UM (HR for death, 2.6; 95% CI, 1.8-3.9; P < .001; Figure 1C). The causes of death are shown in Table 1. Only 16 of 88 (18%) patients with IGHV-M died of causes related to progressive, refractory CLL (defined as death from CLL, Richter transformation, or post-allogeneic stem cell transplantation) vs 69 of 126 (55%) patients with IGHV-UM. Patients with IGHV-M were less likely to die from progressive, refractory CLL (43% of deaths in patients with IGHV-M) than from other causes, which is remarkable, given that almost all relapses in this group of patients occurred before the availability of targeted agents. In contrast, 75% of deaths in patients with IGHV-UM were due to progressive, refractory CLL.
Causes of death in the full cohort and according to IGHV-MS
| Cause of death . | Full cohort, no. of deaths (% of all patients who died from this cause/% of total deaths attributable to this cause) . | IGHV-M, no. of deaths (% of all patients with IGHV-M who died from this cause/% of total deaths in patients with IGHV-M attributable to this cause) . | IGHV-UM, no. of deaths (% of all patients with IGHV-UM who died from this cause/% of total deaths in patients with IGHV-UM attributable to this cause) . |
|---|---|---|---|
| Total deaths | 169 of 300 (56%) | 35 of 88 (40%) | 92 of 126 (73%) |
| CLL | 69 (23/41) | 9 (10/26) | 44 (35/48) |
| Richter transformation | 24 (8/14) | 5 (6/14) | 12 (10/13) |
| Solid tumor | 18 (6/11) | 5 (6/14) | 8 (6/9) |
| Other hematologic neoplasm | 15 (5/9) | 5 (6/14) | 2 (2/2) |
| Infection in remission | 11 (4/7) | 3 (3/9) | 5 (4/5) |
| Nonrelapse mortality after allogeneic SCT | 14 (5/8) | 1 (1/3) | 13 (10/14) |
| Other | 2 (1/1) | 2 (2/6) | 0 (0/0) |
| Unknown | 16 (5/9) | 5 (6/14) | 8 (6/9) |
| Cause of death . | Full cohort, no. of deaths (% of all patients who died from this cause/% of total deaths attributable to this cause) . | IGHV-M, no. of deaths (% of all patients with IGHV-M who died from this cause/% of total deaths in patients with IGHV-M attributable to this cause) . | IGHV-UM, no. of deaths (% of all patients with IGHV-UM who died from this cause/% of total deaths in patients with IGHV-UM attributable to this cause) . |
|---|---|---|---|
| Total deaths | 169 of 300 (56%) | 35 of 88 (40%) | 92 of 126 (73%) |
| CLL | 69 (23/41) | 9 (10/26) | 44 (35/48) |
| Richter transformation | 24 (8/14) | 5 (6/14) | 12 (10/13) |
| Solid tumor | 18 (6/11) | 5 (6/14) | 8 (6/9) |
| Other hematologic neoplasm | 15 (5/9) | 5 (6/14) | 2 (2/2) |
| Infection in remission | 11 (4/7) | 3 (3/9) | 5 (4/5) |
| Nonrelapse mortality after allogeneic SCT | 14 (5/8) | 1 (1/3) | 13 (10/14) |
| Other | 2 (1/1) | 2 (2/6) | 0 (0/0) |
| Unknown | 16 (5/9) | 5 (6/14) | 8 (6/9) |
SCT, stem cell transplantation.
When compared with a US reference population matched for age, sex, and year, the relative survival of subjects with IGHV-UM or missing IGHV status was significantly different from the expected survival for matched cohorts (P < .001 and P = .02, respectively), whereas the survival of subjects with IGHV-M was not significantly different (P = .77; Figure 1C).
End of therapy MRD effectively stratified patients into favorable and adverse-risk groups for PFS within IGHV categories (Figure 1D).
Our analysis confirms the excellent long-term PFS in patients with IGHV-M. Late relapses beyond 10 years did occur, suggesting that periodic follow-up is still required in even those patients who were at low risk and completed therapy >10 years ago. Our 5-year PFS in IGHV-M of 78.2% was higher than that reported in the FCR arm of the CLL8 trial (66.6%). However, this may be explained by a higher proportion of patients who were at early stage in our study (25% in Binet A vs 4% in CLL8) or other unmeasured characteristics.3
Excluding Richter transformation, 96 patients (32%) developed 106 other malignancies (supplemental Table 2): solid tumors other than skin cancers, n = 42 (14%); nonmelanoma skin cancer, n = 34 (11%); myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML), n = 19 (6.3%); other hematologic neoplasms, n = 6 (2%); and melanoma, n = 5 (1.7%). Richter transformation occurred in 29 patients (9.7%). The relationship between nonhematologic cancers and FCR is uncertain, but tMNs such as MDS or AML are likely related to chemotherapy. Among the patients who developed MDS or AML, 16 of 19 (84%) died. Eleven of 14 (79%) of those with available cytogenetic or next-generation sequencing studies demonstrated findings typical of tMNs (chromosome 5 or 7 abnormalities, complex cytogenetics, and TP53 mutations). Recent randomized studies, such as FLAIR, showed numerically higher rates of tMN in patients treated with FCR compared with those treated with ibrutinib plus rituximab.10 No pretreatment characteristics predicted the development of tMN (supplemental Table 3). Understanding which patients are at greatest risk of tMNs could potentially inform treatment decisions through preferential use of targeted agents in these patients. Pre-existing clonal hematopoiesis at the time of chemotherapy for lymphoma is associated with an increased risk of developing tMN later.17 Whether pretreatment analysis for the presence of clonal hematopoiesis could similarly be predictive of tMN development in patients with CLL and guide treatment choice is an important future research question. For now, a careful risk-benefit discussion is warranted when counseling patients regarding FCR, balancing the potential for functional cure vs the risk of tMN.
A limitation of our study is incomplete follow-up of all patients, potentially underestimating the incidence of relapse, late complications (eg, other cancers), and overall mortality. Certain cancers that are easily treated with simple surgical excision (eg, nonmelanoma skin cancers) were likely significantly under-reported by patients and in physician notes. Nonetheless, a significant proportion of the initial patients remained at risk for progression (approximately 20%) or death (approximately 37%) after 15 years of follow-up. With these caveats, we contend that FCR remains an option for patients with IGHV-M, given the median PFS of 14.6 years. In contrast, the markedly shorter PFS for patients with IGHV-UM (median, 4.2 years in our study), with 74% of deaths in this patient group being related to CLL, suggests that these patients should receive targeted agents.
After 6 years of follow-up in the E1912 study, IR use produces a PFS superior to that of FCR even among patients with IGHV-M. However, this duration of follow-up is short, and the risk of late relapse after FCR is low in patients with IGHV-M. It remains to be seen whether a similarly low risk of very late relapse is achieved with continuous ibrutinib therapy. Even if the use of IR achieves similar or superior long-term PFS in patients with IGHV-M, continuous administration of ibrutinib for an extended duration would be required to match the outcomes seen with 6 months of FCR.
Given the concern for long-term fatal toxicities with FCR (tMN) and ibrutinib (ventricular arrhythmias) as well as the high financial burden and risk of developing genetic resistance18 with continuous ibrutinib, we await longer-term PFS data from the CLL13/GAIA study13; if the higher U-MRD rates in the venetoclax plus obinutuzumab–containing arms translate into superior PFS and, perhaps, a cure fraction analogous to that seen with FCR without an increased risk of tMN, time-limited venetoclax plus obinutuzumab–based regimens may become the treatment-of-choice in low genomic-risk CLL. Certainly, longer-term data from CLL14, with a 6-year PFS of approximately 75% among patients with IGHV-M who received venetoclax plus obinutuzumab, are encouraging in this regard.19 Bruton tyrosine kinase inhibitor plus venetoclax doublets are another emerging time-limited frontline therapy with potential for long-term remission, hopefully without increased risk of tMN.20
Acknowledgments
The authors thank Lizette Lopez and Stephanie Zelaya for their assistance with gathering long-term follow-up data.
This work was funded by a grant from the CLL Global Research Foundation.
We dedicate this article to Susan Lerner, who lovingly cared for our CLL database and without whom much of this work would not have been possible.
Authorship
Contribution: P.A.T. coordinated the project, provided clinical care to patients, gathered and analyzed data, and wrote the paper; A.B. gathered and analyzed data and co-wrote the paper; W.G.W. provided clinical care to patients, developed critical themes, and co-wrote the paper; C.S.T. gathered data, provided clinical care to patients, developed critical themes, and co-wrote the paper; S.M.O. provided clinical care to patients, developed critical themes, and co-wrote the paper; S.S. and C.B.P. provided statistical analysis support and co-wrote the paper; W.P. designed the clinical protocol and co-wrote the paper; and M.J.K. designed the clinical protocol, provided clinical care to patients, developed critical themes, and co-wrote the paper.
Conflict-of-interest disclosure: P.A.T. reports consultant fees/honoraria from Adaptive Biotechnologies, AbbVie, AstraZeneca, BeiGene, Genentech, LOXO/Lilly, Janssen, and Pharmacyclics, unrelated to the submitted work; and research funding from Adaptive Biotechnologies, AbbVie, Genentech, LOXO/Lilly, and Pharmacyclics, unrelated to the submitted work. W.G.W. receives research funding from AbbVie; AstraZeneca; Acerta Pharma; Bristol Myers Squibb; Cyclacel; Eli Lilly/Loxo; Genentech; Gilead Sciences; Glaxo Smith Kline/Novartis; Janssen; Juno Therapeutics; Kite, a Gilead company; Miragen; Oncternal Therapeutics; Pharmacyclics LLC, an AbbVie company; Sunesis Pharmaceuticals; and Xencor. C.S.T. has a consultant or advisory role in AbbVie, BeiGene, Janssen, Loxo, and Roche; receives honoraria from AbbVie, BeiGene, Janssen-Cilag, Genentech-Roche, Loxo/Eli Lilly, Novartis, and Pharmacyclics LLC, an AbbVie company; and receives research funding from AbbVie, BeiGene, and Janssen-Cilag. S.M.O. has consulted for AbbVie, Alexion, Amgen, Aptose Biosciences, Astellas, AstraZeneca, Autolus, Bristol Myers Squibb, Celgene DynaMed, Eli Lilly and Company, Gilea, GlaxoSmithKline, Janssen Oncology, Johnson and Johnson, Juno Therapeutics, MEI Pharma, Merck, NOVA Research Company, Pfizer, Pharmacyclics, TG Therapeutics, Vaniam Group, Verastem, and Vida Ventures, and has received research support from Acerta, Alliance, BeiGene, Caribou Biosciences, Gilead, Kite, Loxo Oncology, Mustang, Nurix Theraputics, and Regeneron. The remaining authors declare no competing financial interests.
Correspondence: Philip A. Thompson, Clinical Haematology, Peter MacCallum Cancer Centre, Locked Bag 1, A’Beckett St, Melbourne, VIC 8006, Australia; e-mail: philip.thompson@petermac.org.
References
Author notes
Deidentified individual patient data are available on a case-by-case basis on request from author William G. Wierda (wwierda@mdanderson.org).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal