In this issue of Blood, Wood et al1 report the features and outcome of 1256 children, adolescents, and young adults newly diagnosed with T-cell acute lymphoblastic leukemia (T-ALL) enrolled in the Children Oncology Group, COG AALL0434 study between 2009 and 2014. This study is a critically important evaluation of the impact of contemporary risk-adjusted therapy in early T-cell precursor (ETP) ALL, a biologically and clinically unique subset of T-ALL first defined in 2009.2
T-ALL was previously associated with a poorer outcome than B-lineage ALL and thus treated as high-risk leukemia in many early protocols. Treatment intensification in the last decades has closed the gap with B-lineage ALL, but reliable risk assignment criteria for T-ALL based on genetic abnormalities are lacking. In the past, immunophenotypic features that define maturational stages of T cells in the thymus were applied to T-ALL in efforts to identify prognostically important subtypes, but such classifications (eg, pro-T, pre-T, cortical T, and mature T-ALL) proved to be clinically unhelpful. The strongest prognostic criterion remains early response to treatment as measured by minimal residual disease (MRD).3
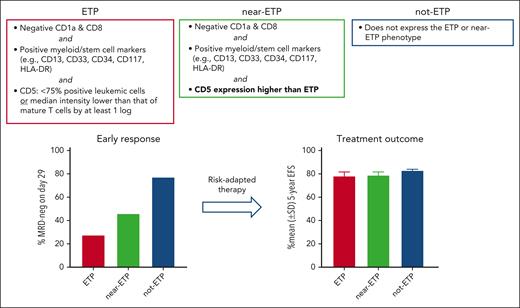
ETP ALL is a distinct subtype of T-ALL that is consistently associated with a poor early treatment response to chemotherapy in pediatric and adult patients.2,4-8 ETP ALL was originally defined by absent CD1a and CD8, weak or negative CD5 (<75% positive leukemic cells or median intensity lower than that of mature T cells by at least 1 log), and expression of myeloid- or stem cell-associated markers.2 This phenotype suggests that ETP ALL cells are the malignant counterpart of immature progenitor cells that have recently migrated to the thymus from the bone marrow.2 ETP ALL also has characteristic genetic features, including activating mutations in genes regulating cytokine receptor signaling and inactivating lesions disrupting hematopoietic development.9 Other subsets of T-ALL that resemble ETP ALL but do not exactly fit the immunophenotypic criteria originally laid out have been identified.2,4,8 In this study, Wood et al used the original ETP ALL criteria, which applied to 11.5% of cases in COG AALL0434. They also studied the clinical features of “near ETP ALL,” defined by the same immunophenotypic criteria of ETP ALL with the exception of a stronger CD5 expression, which included 16.7% of patients in COG AALL0434. Slow treatment response for ETP ALL was confirmed and was also observed for near-ETP cases: 72.4% of patients with ETP ALL had ≥0.1% MRD on day 29 of treatment (≥1% in most cases) vs 54.0% of those with near-ETP ALL and 22.7% of those with not-ETP ALL. Overall outcome of ALL is determined by treatment intensity. The use of MRD as a criterion for intensified therapy, including hematopoietic stem cell transplantation (HSCT), can significantly improve overall treatment outcome when early treatment has been inadequate. Indeed, in the study by Wood et al dramatic differences in early response were not associated with significant differences in event-free and overall survival rates among the 3 groups (see figure). Of note, 27.7% of patients with ETP and near-ETP were classified as having high-risk ALL vs 10.0% of those with not-ETP ALL (P < .0001 by the Fisher exact test) and, hence, received more intensive chemotherapy; 12.7% had HSCT vs 2.3% of those with not-ETP (P < .0001). Also, as stated by Wood et al in reference to COG AALL0434, patients with ETP are more frequently taken off study during induction and referred for HSCT, which may affect conclusions about outcome.
Improved survival in ETP and near-ETP ALL using risk-adapted therapy based on MRD. As reported by Wood et al, patients with ETP and near-ETP ALL achieved 5-year event-free survival equivalent to not-ETP ALL using this approach. EFS, event-free survival; SD, standard deviation.
Improved survival in ETP and near-ETP ALL using risk-adapted therapy based on MRD. As reported by Wood et al, patients with ETP and near-ETP ALL achieved 5-year event-free survival equivalent to not-ETP ALL using this approach. EFS, event-free survival; SD, standard deviation.
The rapid evolution in the understanding of ETP ALL offers a paradigm for combined biological and clinical research initiatives in hematologic malignancies. This comprehensive study by Wood et al provides definitive information on the prevalence and presenting features of ETP and near-ETP ALL and establishes a reference for the rates of expected early response and long-term outcome with contemporary therapy. Although strong prognostic information can be derived from early MRD monitoring, presenting features that predict early treatment response are important for risk assignment and optimizing patient clinical management. In T-ALL, accurate recognition of ETP ALL is critical for early risk assignment, as well as preparation for HSCT and experimental therapy. The study by Wood et al starts a new chapter for ETP ALL. The molecular basis underlying the obvious resistance of ETP ALL cells to chemotherapy still needs to be elucidated, and the biologic differences between ETP- and near-ETP subtypes are still unclear. Further studies in this area might shed light on these issues, provide clues about targetable pathways, and assess whether immunotherapeutic approaches can improve outcome of these chemotherapy-resistant subtypes of ALL.
Conflict-of-interest disclosure: E.C.-S. is coinventor of patents on technologies to monitor MRD; E.C.-S.'s spouse is a scientific founder, consultant, and stockholder of Nkarta Therapeutics and Medisix Therapeutics and a consultant for Locus Cell and Jeito Capital. V.C. has no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal