In this issue of Blood, Leiding et al1 describe outcomes after allogeneic hematopoietic cell transplant (allo-HCT) for patients with chronic granulomatous disease (CGD). This multicenter study confirms that allo-HCT can significantly reduce the disease burden, independent of age, genotype, oxidase status, and history of preceding infections/autoinflammation.1 Increased rates of graft failure (GF) and chronic graft-versus-host disease (GVHD) were seen in patients with reduced performance status, exuberant inflammation before allo-HCT, and human leukocyte antigen (HLA)–mismatched donors.
In 1997, when I was a freshly minted HCT physician, one of my first patients was a 14-year-old boy with X-linked CGD experiencing pulmonary aspergillosis. He underwent myeloablative allo-HCT from his HLA-identical sister after busulfan/cyclophosphamide conditioning. Within 2 weeks of transplant, he was dead of respiratory failure, despite signs of engraftment. What had gone wrong?
CGD is a primary immunodeficiency caused by genetic mutations encoding proteins of the nicotinamide adenine dinucleotide phosphate oxidase complex. This oxidase complex is responsible for the generation of reactive oxygen species in phagocytes, the lysosomal killing of particular bacteria/fungi, and the proper autophagy/efferocytosis of recruited phagocytes. Skin and deep-seated abscesses, lymphadenitis, granulomatous infections, autoinflammation, wasting, and growth failure are common disease manifestations. With life-long antibacterial and antifungal medication and careful use of corticosteroids/biological agents for inflammatory complications, patients with CGD can survive into adulthood.2 Nevertheless, the disease burden increases with age because of progressive organ damage.
During the past 20 years, allo-HCT using sufficiently HLA-matched donors has been curative in patients with CGD but restricted to mainly those with high-risk features, like absent oxidase activity, complicated infections, or corticosteroid-dependent autoinflammation.3-7 Patients with residual oxidase activity and those without HLA-matched donors usually were treated with conventional CGD care, but have increasingly also undergone allo-HCT because of disease progression in the past decade.3,8,9 Previously reported transplant series have shown overall survival (OS) rates >80%, a reduction in infections, a resolution of inflammation, and improvement in quality of life compared with pretransplant.3-7 Nevertheless, transplant-related mortality, GF, and chronic GVHD, seen in approximately one-third of patients, diminished the use of this approach.5-9
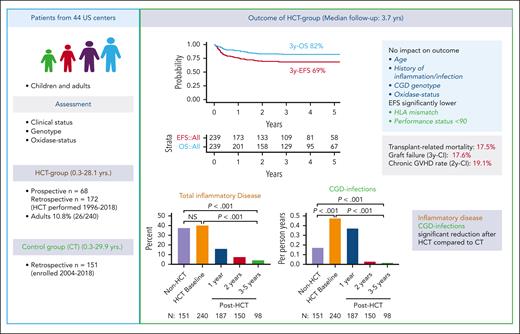
Leiding et al present results of a large cohort of 240 patients with CGD who underwent transplant (HCT), which is the second largest series published after the European cohort published by Chiesa et al (n = 7124). A group of patients with CGD (n = 151) not treated with allo-HCT served as controls (CTs). The overall retrospective character of the analysis and the fact that the allo-HCT cohort (n = 240) included both retrospective (n = 172) and prospective (n = 68) patients rendered a direct comparison between HCT and CT groups of survival and clinical status impossible; nevertheless, useful comparative outcome data could be obtained. The CT group was older, with a median age of 15.7 years, compared with 5 years in the HCT group, and had fewer infections (see Leiding et al, Figure 1A-D and supplemental Figure 1A-B). Close to 90% of allo-HCTs were performed from 2006 to 2018, with the remainder performed from 1996 until 2005 (see figure, left column), similar to the transplant time frames in the study by Chiesa et al.4
HCT alleviates disease burden in CGD. Left column: concept (retrospective and prospective part) of the multicenter study on outcomes after allo-HCT in patients with CGD. Right column, upper part: outcomes with 3-year overall/event-free survival (3y-OS/3y-EFS) and factors influencing outcome after HCT. Lower part: disease burden (total inflammatory disease and CGD infections) at baseline and at 1, 2, and 3 to 5 years after HCT in comparison to the group that did not undergo transplant (Non-HCT). CI, cumulative incidence.
HCT alleviates disease burden in CGD. Left column: concept (retrospective and prospective part) of the multicenter study on outcomes after allo-HCT in patients with CGD. Right column, upper part: outcomes with 3-year overall/event-free survival (3y-OS/3y-EFS) and factors influencing outcome after HCT. Lower part: disease burden (total inflammatory disease and CGD infections) at baseline and at 1, 2, and 3 to 5 years after HCT in comparison to the group that did not undergo transplant (Non-HCT). CI, cumulative incidence.
The 3-year OS/event-free survival rates were 82%/69%, respectively (Chiesa et al: 86.8%/76.4%, respectively4). CGD genotype and oxidase status did not influence outcome, whereas in the report by Chiesa et al, only 40% of patients had genotyping and oxidase status was unavailable.4 Independent of their previous medical history, the disease burden, including infections, inflammation, and nutritional/growth compromise, decreased significantly in engrafted and surviving patients to levels significantly below the HCT and CT baselines (see figure, right column, lower part). A performance status <90% or mismatched donor transplants were associated with poorer outcomes (see figure, right column, upper part). OS of adult patients (aged ≥18 years) who underwent HCT did surprisingly not differ from those who underwent transplant at younger age (see Leiding et al, supplemental Figure 3B-C). Adult’s OS in the cohort of Chiesa et al was clearly inferior to that of children.4 Although the percentage of adults was similar (10.8%) between the study by Leiding et al (26/240) and the study by Chiesa et al (77/7124), the median age at transplant was younger in the study by Leiding et al (5 years; range, 0.3-28.1 years) than in the study by Chiesa et al (7.08 years; range, 0.19-48.6 years4). Whether different performance scores of the pediatric/adult cohorts who underwent transplant accounted for this important difference between US and European patients with CGD remains uncertain.
The study by Leiding et al found that the need for corticosteroids, biological agents, or antimicrobial prophylaxis mostly disappeared in the HCT group compared with pre-HCT and CT baselines (see Leiding et al, Figure 1E-G and supplemental Figure 4), which means cure of the disease. Nevertheless, there was substantial mortality, with 42 patients (17.5%) succumbing to respiratory/multiorgan failure, hemorrhage, GHVD, or infection (a higher rate than reported by Chiesa et al, which was 13%4). The GF rate of 17.6% is within the expected range for CGD but remains a disappointingly high feature of CGD transplant (see figure, right column, upper part) (Chiesa et al: 12.8%4). The rates of II to IV, III to IV acute, and chronic/chronic extensive GVHD, with 18.4% (Chiesa et al: 20.1%4), 6.2% (Chiesa et al: 9%4), and 19.1% (see figure, right column, upper part)/6.7% (Chiesa et al: 17.8%/6.2%4), respectively, show that alloreactivity also remains a problem despite the inclusion of serotherapy in most (92%) of patients (Chiesa et al: 74%4). Differences in donor distribution (ie, 25% matched related donors [Chiesa et al: 36.1%4]), 49.2% matched unrelated donors (Chiesa et al: 40.7%4), 9.2% mismatched related donors (Chiesa et al: 5%4), 16.7% mismatched unrelated donors (Chiesa et al: 15%4), and the use of cord blood (15% vs 4.2%4) render a direct comparison of GF and GVHD rates between both studies difficult; nevertheless, HLA mismatch remains an important factor negatively influencing event-free survival (see Leiding et al, supplemental Figure 3F).4 The use of melphalan-containing conditioning led to more GF, and busulfan-containing regimens (administered in 77% of patients; see Leiding et al, Table 2) yielded better myeloid engraftment. Regular donor chimerism (DC) analysis, especially for the myeloid fraction, was shown to be important to monitor for imminent secondary GF. If myeloid DC was sufficient (≥95%), mixed CD3+ T-cell chimerism was not associated with increased rates of GF or autoimmunity. Serotherapy with alemtuzumab was superior to antithymocyte globulin in preventing GVHD, which is a novel finding and further supports its use in CGD transplant.3,6-8 Therapeutic drug monitoring for alemtuzumab,10 similar to that established for busulfan,3 could help to further reduce the GF in CGD transplant.
Returning to my patient, today I would have reduced toxicity by adding antithymocyte globulin and given a different preparative regimen. Furthermore, I would have administered corticosteroids in addition to antifungals to prevent respiratory failure during engraftment. Unfortunately, I cannot turn back time. Inspired by the investigation of Leiding et al, all patients with CGD could benefit from cellular therapies in the future, but there remain—surmountable—hurdles to overcome before that becomes a reality.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal