In this issue of Blood, Kameda et al show that aggressive natural killer cell leukemia (ANKL) cells primarily engraft and proliferate in the liver and suggest that the transferrin–transferrin receptor 1 (TfR1) axis may be a new therapeutic target.1
ANKL is a rare hematological malignancy characterized by a short-term clinical course and a particularly poor prognosis. It has a higher prevalence in Asia and South America than in western countries. It is frequently associated with reactivation of Epstein-Barr virus, in addition to the abnormal expansion of mature natural killer cells.2,3 Although the ontogeny of the disease is poorly understood, 3 major groups of molecular abnormalities are found: (1) activating mutations of the JAK/STAT pathway, (2) mutations resulting in epigenetic dysregulation, and (3) impairment of DNA repair or alterations in TP53. Chemotherapy is the standard treatment but only affords a median survival of 2 to 7 months. Allogenic stem cell transplantation extends the survival of some patients, but the overall success is limited.2-4 There is an urgent need for novel therapies and for preclinical models to test their efficacy.
Kameda et al established patient-derived xenograft (PDX) mouse models that have infiltration of ANKL cells in the bone marrow, spleen, and liver. In vivo imaging and flow cytometric analysis of the PDXs revealed that the ANKL cells primarily engraft and proliferate in the liver sinusoids and in the periportal areas of the liver before spreading to the spleen, blood, and bone marrow. They confirmed the sinusoid and/or periportal distribution of ANKL in the liver both in the mouse model and in patients.1 Hepatosplenomegaly and liver dysfunction are also frequently observed in patients with ANKL.3,4 Liver-derived ANKL cells also showed a particularly aggressive phenotype when compared with spleen-derived ANKL from the PDXs, and this was associated with a high expression of MYC and Myc-regulated genes. Analysis of RNA-sequencing data and an interactome analysis between liver cells and ANKL revealed an upregulated expression of the Myc-regulated TfR1 gene in ANKL cells and suggested that iron uptake via the transferrin-TfR1 axis might represent a potential therapeutic vulnerability (see figure).1
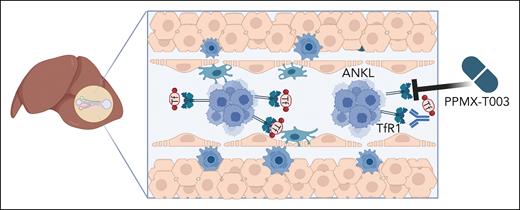
Targeting iron uptake as a new therapeutic option for ANKL. ANKL cells primarily engraft and proliferate in the liver sinusoid. The liver stores iron, and ANKL cells upregulate the TfR1 to take up iron-loaded transferrin. Inhibiting TfR1 by the monoclonal blocking antibody PPMX-T003 lowers iron import and deprives ANKL cells of their iron content, reducing their survival and/or proliferation. Figure created with BioRender.com.
Targeting iron uptake as a new therapeutic option for ANKL. ANKL cells primarily engraft and proliferate in the liver sinusoid. The liver stores iron, and ANKL cells upregulate the TfR1 to take up iron-loaded transferrin. Inhibiting TfR1 by the monoclonal blocking antibody PPMX-T003 lowers iron import and deprives ANKL cells of their iron content, reducing their survival and/or proliferation. Figure created with BioRender.com.
The TfR1 is a ubiquitously expressed membrane glycoprotein that mediates the cellular uptake of iron from the plasma glycoprotein transferrin. Iron uptake involves the binding of transferrin to the transferrin receptor, followed by receptor-mediated endocytosis.5 Iron is stored in the liver and is a critical element in several cellular functions, such as oxygen transport, DNA synthesis and repair, and, thereby, cell proliferation. There is accumulating evidence that iron metabolism plays a crucial role in cancer progression, and many tumor cells show a higher dependency on iron than healthy cells.6,7 Kameda et al performed an in vivo CRISPR-Cas9 manipulation of the ANKL PDXs to confirm that ANKL cells are vulnerable to iron deprivation and that the transferrin-TfR1 interaction between liver cells and ANKL is a potential therapeutic target. Treatment of the ANKL PDXs with PPMX-T003, a humanized anti-TfR1 monoclonal antibody, resulted in a significantly enhanced disease latency and in eradication of the disease from the liver, although lesions remained in spleen and bone marrow.
Novel therapeutic approaches, such as immune checkpoint inhibitors and cellular immune therapy, and targeted approaches (eg, inhibiting the JAK/STAT signaling pathway) are currently being explored for the treatment of patients with ANKL.3 PPPMX-T003 showed an acceptable safety profile in a phase 1 clinical trial.8 The study by Kameda et al provides important insights into the iron metabolism of ANKL and provides justification for further clinical trials based on the idea of targeting iron uptake via the TfR1 receptor axis to treat this fatal disease.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal