Key Points
Male survivors of HL treated with chest radiotherapy have an increased risk of developing BC compared with the general population.
Although the occurrence of male BC is an uncommon event, clinicians should be alert to BC symptoms in male survivors of HL.
Abstract
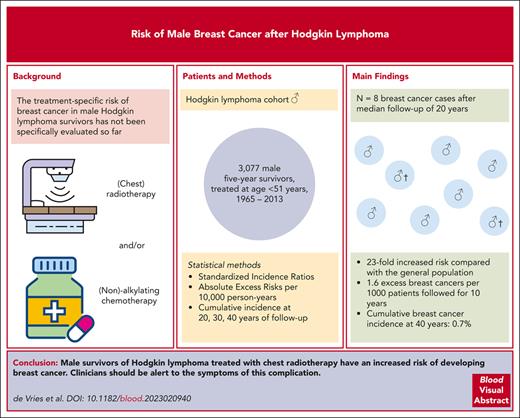
Female survivors of Hodgkin lymphoma (HL) treated with chest radiotherapy have a strongly increased risk of breast cancer (BC), but the treatment-specific BC risk in male survivors of HL has not been evaluated. We assessed BC risk in a cohort of 3077 male survivors of 5-year HL treated at age ≤51 years in 20 Dutch hospitals between 1965 and 2013. We estimated standardized incidence ratios (SIRs), absolute excess risks per 10 000 person-years, and cumulative BC incidences. After a 20-year median follow-up, we observed 8 cases of male with BC. Male survivors of HL experienced a 23-fold (95% confidence interval [CI], 10.1-46.0) increased BC risk compared with the general population, representing 1.6 (95% CI, 0.7-3.3) excess BC incidences per 10 000 person-years. The 20- and 40-year cumulative BC incidences after HL treatment were 0.1% (95% CI, 0.02-0.3) and 0.7% (95% CI, 0.3-1.4), respectively. Treatment with chest radiotherapy without alkylating chemotherapy yielded a strongly increased SIR (20.7; 95% CI, 2.5-74.8), which was not significantly different for chest radiotherapy and alkylating chemotherapy (41.1; 95% CI, 13.4-96.0). Males treated with chest radiotherapy and anthracyclines had an SIR of 48.1 (95% CI, 13.1-123.1). Two patients died from BC (median follow-up, 4.7 years). To ensure early diagnosis and treatment, clinicians should be alert to BC symptoms in male survivors of HL.
Introduction
Numerous studies have shown that female survivors of Hodgkin lymphoma (HL) treated with chest radiotherapy have an increased breast cancer (BC) risk.1-4 The risk increases with higher radiation doses, larger volumes, and younger age at treatment.4-9 Treatment-induced primary ovarian insufficiency appears to reduce radiation-associated risk.5,10 Because male BC is a rare disease in the general population, very few studies assessed the risk of BC in male survivors of HL. Although several large HL cohort studies observed at least 1 case of male BC, we identified only 2 studies that quantified the risk of male BC after HL, both restricted to childhood survivors of HL.11,12 So far, no studies provided treatment-specific risk estimates. Therefore, we assessed the treatment-specific risk of BC in males in a large cohort of Dutch survivors of HL with long-term and near complete follow-up.
Study design
Our cohort comprised 3077 male survivors of 5-year HL treated before age 51 years between 1965 and 2013 in 7 university and 13 general hospitals in the Netherlands. Detailed information regarding HL diagnosis, treatment modalities of primary and relapse treatment (ie, radiation fields, chemotherapy regimens, and number of cycles), and follow-up was collected from medical records as described previously.13-16 Data on subsequent primary cancers were collected via review of medical records, responses to questionnaires sent to general practitioners (the response regarding subsequent malignancies was 96% complete until 1989),17 and record linkage with the Netherlands Cancer Registry (NCR) since 1989, when the NCR reached nationwide coverage.18,19
The time of being at risk was calculated starting at 5 years after the start of HL treatment and ended at date of the first BC diagnosis, death, or censoring (date of last vital status in case of linkage with NCR or date of last medical information otherwise), whichever occurred first. None of the male survivors of HL developed a ductal carcinoma in situ of the breast. Subsequent primary cancers other than BC were ignored in analyses. Expected numbers of BC incidences were calculated based on age-specific and calendar period–specific male BC incidence rates in the Dutch population multiplied by the corresponding number of at-risk person-years. From the observed and expected numbers of BCs, we calculated standardized incidence ratios (SIRs), absolute excess risks (AERs; expressed per 10 000 person-years), and the corresponding 95% confidence intervals (CIs).20 Tests for heterogeneity and trends of SIR by age at HL treatment were performed within collapsed person-time in Poisson regression models.21 Cumulative 20-, 30-, and 40-year BC incidence was estimated in the presence of death as a competing risk. P < .05 was considered statistically significant. All statistical tests were performed using Stata statistical software, version 15 (StataCorp, College Station, TX).
Results and discussion
After a median follow-up of 20 years (interquartile range [IQR], 14-26 years), we observed 8 males with BC in our cohort of survivors of HL (characteristics presented in Table 1). The median age at BC diagnosis was 55 years (IQR, 46-58 years), and the median interval between start of HL treatment and BC diagnosis was 27 (IQR, 21-29; range, 18-32) years. Compared with the general population, BC risk was 23.3-fold increased (95% CI, 10.1-46.0), representing 1.6 (95% CI, 0.7-3.3) excess BCs per 10 000 person-years (Table 2). BC risk decreased with older age at HL treatment (Ptrend = .009); the SIR was highest for males treated aged <25 years (SIR 69.0; 95% CI, 14.2-201.6), corresponding to 1.6 excess cases per 10 000 person-years (95% CI, 0.3-4.9). Risk was not significantly increased in patients treated at age ≥35 years. SIRs remained increased up to 30 years after HL treatment. Regarding attained age at end of follow-up, we also observed the highest SIR among males while they were young (54.8; 95% CI, 1.4-305.6 for those aged <40 years). However, males with an attained age between 50 and 59 years had the highest AER (4.6; 95% CI, 1.4-10.9). However, numbers of events in subgroup analyses were small. The overall cumulative BC incidence was 0.1% (95% CI, 0.02-0.3) at 20 years of follow-up since HL treatment, 0.6% (95% CI, 0.2-1.1) at 30 years, and 0.7% (95% CI, 0.3-1.4) at 40 years. By the age 30 years, 0.04% of male survivors of HL had developed BC (95% CI, 0.004-0.2), and by the age of 60 years, this was 0.4% (95% CI, 0.2-0.8).
Characteristics of subsequent male patients with BC
| Patient no. . | Age at HL (y) . | HL treatment year . | HL treatment regimen . | Interval between HL and BC (y) . | Type of BC . | Primary treatment for BC . | Follow-up time after BC diagnosis (y) . | Vital status at the end of follow-up . |
|---|---|---|---|---|---|---|---|---|
| 1 | 35 | 1973 | RT: mantle field, inverted-Y and splenic hilum (primary; all fields 40 Gy∗) CT: vinblastine as maintenance therapy for ∼2 years | 23 | Infiltrating ductal carcinoma (C50.9, M8500/3) | Surgery and endocrine therapy | 13 | Alive |
| 2† | 23 | 1982 | CT: 8 cycles of MOPP (primary), 4 cycles of MOPP and 6 cycles of MOPP-ABV (relapse) RT: neck, supraclavicular, infraclavicular, and left axillary fields (relapse; RT dose not available) | 18 | Infiltrating duct carcinoma of the left breast (C50.9, M8500/3), TNM-stage IIIA, and ER+/PR+/HER2 equivocal | CT (FAC [5-FU, doxorubicin, and cyclophosphamide]), mastectomy, RT, and endocrine therapy (tamoxifen) | 3 | Deceased (cause of death: BC) |
| 3‡ | 32 | 1978 | CT: 6 cycles of MOPP (primary) RT: Inverted-Y, spleen, and inguinal field (primary; all fields 40 Gy∗) | 32 | Infiltrating duct carcinoma of the left breast (C50.9, M8500/3), TNM-stage IIB, and ER+/PR+/HER2– | Endocrine therapy (exemestane, tamoxifen, and anastrozole) | 6 | Deceased (cause of death: BC) |
| 4§ | 28 | 1993 | CT: 6 cycles of MOPP-ABV (primary) RT: mediastinum, supraclavicular, and para-aortic fields (primary; RT dose not available) | 30 | Infiltrating duct carcinoma of the left breast (C50.9, M8500/3) | Surgery only | 0 | Alive |
| 5 | 12 | 1991 | CT: 2 cycles of OEPA and 2 cycles of COPP (primary) RT: neck, supraclavicular, infraclavicular, and right axillary fields (primary; RT dose not available) | 27 | Infiltrating duct carcinoma of the left breast (C50.1, M8500/3), TNM-stage IIA, and ER+/PR+/HER2– | CT (AC [doxorubicin/cyclo-phosphamide] and docetaxel), surgery, RT, and endocrine therapy (tamoxifen) | 4 | Alive |
| 6‖,¶ | 38 | 1998 | CT: 6 cycles of MOPP-ABV (primary) RT: mediastinum, neck, left lung, and para-aortic field, and spleen (primary; 20 Gy to the left lung, fields below diaphragm 30 Gy, other fields above diaphragm 40 Gy∗) | 18 | Infiltrating duct carcinoma of the left breast (C50.1, M8500/3), TNM-stage IIA, and ER+/PR+/HER2+ | CT (taxanes), targeted therapy (trastuzumab), surgery, and endocrine therapy (tamoxifen) | 6 | Alive |
| 7∗∗ | 25 | 1994 | RT: mediastinum, neck, supraclavicular, and para-aortic field, and spleen (primary; neck and supraclavicular fields 40 Gy, other fields 36 Gy∗) | 27 | Infiltrating duct carcinoma (C50.4, M8500/3), TNM-stage IA, and ER+/PR+/HER2– | Breast conserving surgery and endocrine therapy (tamoxifen) | 2 | Deceased (cause of death: lung cancer) |
| 8 | 26 | 1979 | RT: mantle field, inverted-Y, and spleen (primary; RT dose not available) CT: 8 cycles of BCVPP (carmustine, cyclophosphamide, vinblastine, procarbazine, and prednisone) (relapse) | 27 | Infiltrating duct carcinoma of the left breast (C50.1, M8500/3), TNM-stage IA, ER+/PR+/HER2– | Mastectomy only | 7 | Alive |
| Patient no. . | Age at HL (y) . | HL treatment year . | HL treatment regimen . | Interval between HL and BC (y) . | Type of BC . | Primary treatment for BC . | Follow-up time after BC diagnosis (y) . | Vital status at the end of follow-up . |
|---|---|---|---|---|---|---|---|---|
| 1 | 35 | 1973 | RT: mantle field, inverted-Y and splenic hilum (primary; all fields 40 Gy∗) CT: vinblastine as maintenance therapy for ∼2 years | 23 | Infiltrating ductal carcinoma (C50.9, M8500/3) | Surgery and endocrine therapy | 13 | Alive |
| 2† | 23 | 1982 | CT: 8 cycles of MOPP (primary), 4 cycles of MOPP and 6 cycles of MOPP-ABV (relapse) RT: neck, supraclavicular, infraclavicular, and left axillary fields (relapse; RT dose not available) | 18 | Infiltrating duct carcinoma of the left breast (C50.9, M8500/3), TNM-stage IIIA, and ER+/PR+/HER2 equivocal | CT (FAC [5-FU, doxorubicin, and cyclophosphamide]), mastectomy, RT, and endocrine therapy (tamoxifen) | 3 | Deceased (cause of death: BC) |
| 3‡ | 32 | 1978 | CT: 6 cycles of MOPP (primary) RT: Inverted-Y, spleen, and inguinal field (primary; all fields 40 Gy∗) | 32 | Infiltrating duct carcinoma of the left breast (C50.9, M8500/3), TNM-stage IIB, and ER+/PR+/HER2– | Endocrine therapy (exemestane, tamoxifen, and anastrozole) | 6 | Deceased (cause of death: BC) |
| 4§ | 28 | 1993 | CT: 6 cycles of MOPP-ABV (primary) RT: mediastinum, supraclavicular, and para-aortic fields (primary; RT dose not available) | 30 | Infiltrating duct carcinoma of the left breast (C50.9, M8500/3) | Surgery only | 0 | Alive |
| 5 | 12 | 1991 | CT: 2 cycles of OEPA and 2 cycles of COPP (primary) RT: neck, supraclavicular, infraclavicular, and right axillary fields (primary; RT dose not available) | 27 | Infiltrating duct carcinoma of the left breast (C50.1, M8500/3), TNM-stage IIA, and ER+/PR+/HER2– | CT (AC [doxorubicin/cyclo-phosphamide] and docetaxel), surgery, RT, and endocrine therapy (tamoxifen) | 4 | Alive |
| 6‖,¶ | 38 | 1998 | CT: 6 cycles of MOPP-ABV (primary) RT: mediastinum, neck, left lung, and para-aortic field, and spleen (primary; 20 Gy to the left lung, fields below diaphragm 30 Gy, other fields above diaphragm 40 Gy∗) | 18 | Infiltrating duct carcinoma of the left breast (C50.1, M8500/3), TNM-stage IIA, and ER+/PR+/HER2+ | CT (taxanes), targeted therapy (trastuzumab), surgery, and endocrine therapy (tamoxifen) | 6 | Alive |
| 7∗∗ | 25 | 1994 | RT: mediastinum, neck, supraclavicular, and para-aortic field, and spleen (primary; neck and supraclavicular fields 40 Gy, other fields 36 Gy∗) | 27 | Infiltrating duct carcinoma (C50.4, M8500/3), TNM-stage IA, and ER+/PR+/HER2– | Breast conserving surgery and endocrine therapy (tamoxifen) | 2 | Deceased (cause of death: lung cancer) |
| 8 | 26 | 1979 | RT: mantle field, inverted-Y, and spleen (primary; RT dose not available) CT: 8 cycles of BCVPP (carmustine, cyclophosphamide, vinblastine, procarbazine, and prednisone) (relapse) | 27 | Infiltrating duct carcinoma of the left breast (C50.1, M8500/3), TNM-stage IA, ER+/PR+/HER2– | Mastectomy only | 7 | Alive |
ABV, doxorubicin, bleomycin, and vinblastine; BCVPP, bleomycin, cyclophosphamide, vinblastine, procarbazine, and prednisone; COPP, cyclophosphamide, vincristine, procarbazine, and prednisone; CT, chemotherapy; ER, estrogen receptor; FU, fluorouracil; HER2, human epidermal growth factor receptor 2; MOPP, mechlorethamine, vincristine, procarbazine, and prednisone; NOS, not otherwise specified; OEPA, vincristine, etoposide, prednisone, and doxorubicin; PR, progesterone receptor; RT, radiotherapy; TNM, TNM classification of malignant tumors.
Radiotherapy was usually applied in fractions from 1.5 to 2.0 Gy.
Patient developed a malignant tumor of lymphatic tissue NOS in 1999, for which he received chemotherapy.
According to the treating radiation oncologist the BC was estimated to be in the previously irradiated para-aortic and spleen fields.
Patient developed a renal cell carcinoma NOS (C64.9, M8312/3) in 2006, for which he received surgery.
Patient developed a transitional cell, papillary carcinoma in the lateral wall of the bladder (C67.2, M8130/3) in 2017, for which he received surgery and immunotherapy.
Patient developed a basal cell carcinoma of the skin (C44.5, M8090/3) in 2021.
Patient developed an adenocarcinoma of the main bronchus (C34.0, M8140/3) in 2021, for which he received radiotherapy and chemotherapy.
SIRs and AERs of subsequent BC incidence in males
| Characteristic . | No. of patients (%) . | Male BC cases . | SIR (95% CI) . | AER (95% CI) . |
|---|---|---|---|---|
| Overall | 3077 (100) | 8 | 23.3 (10.1-46.0) | 1.6 (0.7-3.3) |
| Age at HL diagnosis, y | ||||
| <25 | 1036 (33.7) | 3 | 69.0 (14.2-201.6) | 1.6 (0.3-4.9) |
| 25-34 | 1022 (33.2) | 4 | 43.5 (11.8-111.3) | 2.5 (0.6-6.4) |
| 35-50 | 1019 (33.1) | 1 | 4.8 (0.1-26.9) | 0.6 (−0.1 to 4.1) |
| Treatment period | ||||
| 1965-1979 | 623 (20.3) | 2 | 20.6 (2.5-74.4) | 1.5 (0.1-5.8) |
| 1980-1995 | 1325 (43.1) | 5 | 31.2 (10.1-72.9) | 2.2 (0.7-5.3) |
| 1996-2013 | 1129 (36.7) | 1 | 11.7 (0.3-65.2) | 0.7 (−0.05 to 4.2) |
| Follow-up after HL treatment/interval HL treatment and male BC, y | ||||
| 5-9 | 431 (14.0) | 0 | — | — |
| 10-19 | 1120 (36.4) | 2 | 14.6 (1.8-52.9) | 0.9 (0.04-3.3) |
| 20-29 | 1094 (35.6) | 5 | 42.1 (13.7-98.3) | 5.3 (1.6-12.4) |
| ≥30 | 432 (14.0) | 1 | 23.1 (0.6-128.6) | 4.8 (−0.1 to 27.5) |
| Attained age at end of follow-up, y | ||||
| <40 | 567 (18.4) | 1 | 54.8 (1.4-305.6) | 0.6 (0.004-3.2) |
| 40-49 | 878 (28.5) | 1 | 18.4 (0.5-102.6) | 0.6 (−0.02 to 3.7) |
| 50-59 | 938 (30.5) | 5 | 40.4 (13.1-94.3) | 4.6 (1.4-10.9) |
| ≥60 | 694 (22.3) | 1 | 6.8 (0.2-38.1) | 2.0 (−0.3 to 12.8) |
| HL treatment | ||||
| RT alone | 683 (22.2) | 1 | 10.1 (0.3-56.1) | 0.7 (−0.1 to 4.4) |
| CT alone | 402 (13.1) | 0 | — | — |
| RT + CT | 1992 (64.7) | 7 | 35.3 (14.2-72.8) | 2.3 (0.9-4.9) |
| HL treatment according to chest RT∗and alkylating CT† | ||||
| Chest RT (with or without other RT fields) only | 549 (17.8) | 1 | 12.7 (0.3-70.6) | 0.9 (−0.1 to 5.4) |
| Chest RT (with or without other RT fields) and nonalkylating CT | 151 (4.9) | 1 | 56.5 (1.4-315.0) | 3.4 (0.03-19.1) |
| Chest RT (with or without other RT fields) and alkylating CT | 1352 (43.9) | 5 | 41.1 (13.4-96.0) | 2.5 (0.8-6.0) |
| Other RT fields and alkylating CT | 433 (14.1) | 1 | 19.0 (0.5-106.1) | 1.6 (−.0.4 to 9.1) |
| Other RT fields with or without nonalkylating CT | 190 (6.2) | 0 | — | — |
| CT only‡ | 402 (13.1) | 0 | — | — |
| HL treatment according to chest RT∗ and anthracycline-containing CT | ||||
| Chest RT (with or without other RT fields) only | 549 (17.8) | 1 | 12.7 (0.3-70.6) | 0.9 (−0.1-5.4) |
| Chest RT (with or without other RT fields) and no anthracycline-containing CT | 471 (15.3) | 2 | 35.7 (4.3-128.9) | 2.4 (0.2-8.7) |
| Chest RT (with or without other RT fields) and anthracycline-containing CT | 1032 (33.5) | 4 | 48.1 (13.1-123.1) | 2.8 (0.7-7.3) |
| Other RT fields and anthracycline-containing CT | 390 (12.7) | 0 | — | — |
| Other RT fields with or without no anthracycline-containing CT | 233 (7.6) | 1 | 28.4 (0.7-158.2) | 2.3 (−0.02 to 13.1) |
| CT only‡ | 402 (13.1) | 0 | — | — |
| Characteristic . | No. of patients (%) . | Male BC cases . | SIR (95% CI) . | AER (95% CI) . |
|---|---|---|---|---|
| Overall | 3077 (100) | 8 | 23.3 (10.1-46.0) | 1.6 (0.7-3.3) |
| Age at HL diagnosis, y | ||||
| <25 | 1036 (33.7) | 3 | 69.0 (14.2-201.6) | 1.6 (0.3-4.9) |
| 25-34 | 1022 (33.2) | 4 | 43.5 (11.8-111.3) | 2.5 (0.6-6.4) |
| 35-50 | 1019 (33.1) | 1 | 4.8 (0.1-26.9) | 0.6 (−0.1 to 4.1) |
| Treatment period | ||||
| 1965-1979 | 623 (20.3) | 2 | 20.6 (2.5-74.4) | 1.5 (0.1-5.8) |
| 1980-1995 | 1325 (43.1) | 5 | 31.2 (10.1-72.9) | 2.2 (0.7-5.3) |
| 1996-2013 | 1129 (36.7) | 1 | 11.7 (0.3-65.2) | 0.7 (−0.05 to 4.2) |
| Follow-up after HL treatment/interval HL treatment and male BC, y | ||||
| 5-9 | 431 (14.0) | 0 | — | — |
| 10-19 | 1120 (36.4) | 2 | 14.6 (1.8-52.9) | 0.9 (0.04-3.3) |
| 20-29 | 1094 (35.6) | 5 | 42.1 (13.7-98.3) | 5.3 (1.6-12.4) |
| ≥30 | 432 (14.0) | 1 | 23.1 (0.6-128.6) | 4.8 (−0.1 to 27.5) |
| Attained age at end of follow-up, y | ||||
| <40 | 567 (18.4) | 1 | 54.8 (1.4-305.6) | 0.6 (0.004-3.2) |
| 40-49 | 878 (28.5) | 1 | 18.4 (0.5-102.6) | 0.6 (−0.02 to 3.7) |
| 50-59 | 938 (30.5) | 5 | 40.4 (13.1-94.3) | 4.6 (1.4-10.9) |
| ≥60 | 694 (22.3) | 1 | 6.8 (0.2-38.1) | 2.0 (−0.3 to 12.8) |
| HL treatment | ||||
| RT alone | 683 (22.2) | 1 | 10.1 (0.3-56.1) | 0.7 (−0.1 to 4.4) |
| CT alone | 402 (13.1) | 0 | — | — |
| RT + CT | 1992 (64.7) | 7 | 35.3 (14.2-72.8) | 2.3 (0.9-4.9) |
| HL treatment according to chest RT∗and alkylating CT† | ||||
| Chest RT (with or without other RT fields) only | 549 (17.8) | 1 | 12.7 (0.3-70.6) | 0.9 (−0.1 to 5.4) |
| Chest RT (with or without other RT fields) and nonalkylating CT | 151 (4.9) | 1 | 56.5 (1.4-315.0) | 3.4 (0.03-19.1) |
| Chest RT (with or without other RT fields) and alkylating CT | 1352 (43.9) | 5 | 41.1 (13.4-96.0) | 2.5 (0.8-6.0) |
| Other RT fields and alkylating CT | 433 (14.1) | 1 | 19.0 (0.5-106.1) | 1.6 (−.0.4 to 9.1) |
| Other RT fields with or without nonalkylating CT | 190 (6.2) | 0 | — | — |
| CT only‡ | 402 (13.1) | 0 | — | — |
| HL treatment according to chest RT∗ and anthracycline-containing CT | ||||
| Chest RT (with or without other RT fields) only | 549 (17.8) | 1 | 12.7 (0.3-70.6) | 0.9 (−0.1-5.4) |
| Chest RT (with or without other RT fields) and no anthracycline-containing CT | 471 (15.3) | 2 | 35.7 (4.3-128.9) | 2.4 (0.2-8.7) |
| Chest RT (with or without other RT fields) and anthracycline-containing CT | 1032 (33.5) | 4 | 48.1 (13.1-123.1) | 2.8 (0.7-7.3) |
| Other RT fields and anthracycline-containing CT | 390 (12.7) | 0 | — | — |
| Other RT fields with or without no anthracycline-containing CT | 233 (7.6) | 1 | 28.4 (0.7-158.2) | 2.3 (−0.02 to 13.1) |
| CT only‡ | 402 (13.1) | 0 | — | — |
Bold numbers indicate statistically significant SIRs and AERs (P < .05).
CT, chemotherapy; MOPP, mechlorethamine, vincristine, procarbazine, and prednisone; RT, radiotherapy.
Chest radiotherapy was defined as mantle field RT or RT to the mediastinum, lungs, or axilla.
Alkylating chemotherapy consists of combinations of cytostatic agents with at least 1 alkylating agent (ie, procarbazine, cyclophosphamide, ifosfamide, lomustine, melphalan, dacarbazine, cisplatin, mechlorethamine, chlorambucil, or carmustine).
CT-only category includes patients who have been treated with chemotherapy only, mainly including doxorubicin, bleomycin, vinblastine, and dacarbazine, or MOPP, doxorubicin, bleomycin, and vinblastine.
So far, data on BC risk in male survivors of HL are scarce. Recently, a systematic review on male BC after childhood cancer concluded that most reports on male BC concerned case reports and that risk measures were only reported in 5 cohort studies of pediatric cancer survivors.12 The authors conducted an analysis in the PanCareSurFup childhood cancer cohort and reported a 35.8-fold increased BC risk (95% CI, 7.4-104.6, based on 3 cases) in male survivors of childhood HL compared with the general population, corresponding to an excess of 0.5 cases per 10 000 person-years (95% CI, 0.1-1.4). We observed a lower SIR but a higher AER, which can be explained by a difference in background risk because survivors of HL in our study had a median attained age of 50.9 years, compared with 33.6 years in the PanCareSurFup cohort. There was no difference in duration of follow-up (median, 20 years).
To our knowledge, our study is the first report to assess treatment-specific risks for male BC. Based on research in female survivors of HL, we know that chest radiotherapy, especially with large radiation volumes and high radiation doses, strongly increases BC risk.2 However, treatment with alkylating chemotherapy reduces BC risk, which has been attributed to primary ovarian insufficiency. In our cohort, 7 of the 8 survivors of HL with BC were treated with chest radiotherapy (including axillary fields), and 5 of these men had received alkylating chemotherapy. In line with the findings in women, treatment with chest radiotherapy without alkylating chemotherapy yielded a strongly increased SIR (20.7; 95% CI, 2.5-74.8); the SIR after treatment with chest radiotherapy and alkylating chemotherapy was not significantly different (41.1; 95% CI, 13.4-96.0).
Recent data among female childhood cancer survivors show an increased BC risk after anthracycline-containing chemotherapy.6,22-25 Four males with BC received anthracycline-containing chemotherapy for their HL, all of whom also received chest radiotherapy. We did not observe BC cases in patients who were treated with anthracyclines without chest radiotherapy. Treatment with chest radiotherapy and anthracycline-containing chemotherapy resulted in a SIR of 48.1 (95% CI, 13.1-123.1). Because of the limited number of cases, it was not possible to examine separate effects of chest radiotherapy, anthracyclines, and alkylating agents.
The majority of males were diagnosed with stage II BC. Almost all males were treated for BC with radiotherapy, chemotherapy, and/or endocrine therapy before or after surgery (Table 1). Remarkably, 3 males had already developed another malignancy before BC. Two males developed a malignancy after BC, of whom 1 also had another malignancy before (Table 1). After a median follow-up of 4.7 years (IQR, 2.6-6.3 years) since BC diagnosis, 2 males had died from BC.
In conclusion, this study shows a 23-fold increased BC risk in male survivors of HL up to 30 years after HL treatment compared with that in the general population. Because of the rarity of male BC in the general population, this high relative risk results in only 1.6 excess BC cases per 1000 patients followed up for 10 years. Although male BC after HL treatment is an uncommon event, with a 40-year cumulative incidence of 0.7%, clinicians should be aware of this strongly increased risk, particularly in patients who were treated with chest radiotherapy and had a young age at HL treatment. To ensure early diagnosis and treatment, clinicians should be alert to symptoms of BC in male survivors of HL.
Acknowledgments
The authors thank Rosemarie Wijnands for the data support at the Netherlands Cancer Institute.
This work was supported by the Dutch Cancer Society (NKI 2010-4720) (F.E.v.L.).
Authorship
Contribution: S.d.V., I.M.K., B.M.P.A., and F.E.v.L. designed the study; C.P.M.J., S.E.R., J.M.R., M.R.N., Y.M.B., B.M.P.A., and the BETER consortium provided study patients; S.d.V., M.S., and F.E.v.L. collected and assembled data; S.d.V., I.M.K., M.S., B.M.P.A., and F.E.v.L. analyzed and interpreted data; S.d.V., I.M.K., and F.E.v.L. contributed to manuscript writing; and all authors approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the BETER (Better care after Hodgkin lymphoma: Evaluation of long-term Treatment Effects and screening Recommendations) consortium appears in the supplemental Appendix.
Correspondence: Flora E. van Leeuwen, Department of Epidemiology, The Netherlands Cancer Institute, Plesmanlaan 121, 1066 CX Amsterdam, The Netherlands; e-mail: f.v.leeuwen@nki.nl.
References
Author notes
∗S.d.V., I.M.K., B.M.P.A., and F.E.v.L. contributed equally to this study.
The data underlying this article will be available on reasonable request from the corresponding author, Flora E. van Leeuwen (f.v.leeuwen@nki.nl).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal