Background: Diamond-Blackfan anemia (DBA) is a heritable bone marrow failure disorder characterized by macrocytic anemia with reticulocytopenia and bone marrow erythroid hypoplasia, usually presenting within the first two years of life. Treatment consists of systemic corticosteroids. Treatment failure or steroid intolerance occurs in approximately half of all cases, necessitating chronic transfusions, which carry the risks of iron overload and infections. Hematopoietic cell transplant remains the sole curative therapy for DBA.
In cases where a causative mutation can be identified, with rare exceptions, the affected genes encode ribosomal proteins (RPs), defining DBA as prototypic haploinsufficient ribosomopathy. Erythrocyte hemoglobinization requires balanced heme synthesis and globin translation. Various in vitro and animal models suggest that RP insufficiency results in reduced ribosomal biogenesis, disrupting this balance and leading to excess of heme, resulting in toxicity to developing erythroid cells and premature termination of erythroid differentiation.
Bitopertin is an investigational, orally administered, inhibitor of the GlyT1 glycine transporter. Outside the central nervous system (CNS), GlyT1 is expressed almost exclusively on developing erythrocytes. By reducing intracellular glycine, a rate-limiting substrate for heme synthesis, bitopertin slows heme synthesis and thus we hypothesize rebalances hemoglobin synthesis. In preclinical DBA models, bitopertin rescues erythropoiesis. The drug was originally developed for CNS indications, and has been shown to exhibit a favorable safety profile in prior studies with cumulative enrollment of over 4,000 patients and healthy volunteers. It is currently under clinical investigation for the treatment of erythropoietic protoporphyria.
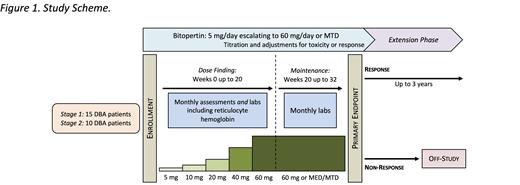
Study Design and Methods: This is a phase I/II, intra-patient dose-escalation study of bitopertin for steroid-refractory DBA, conducted at the National Institutes of Health (NIH) Clinical Center in Bethesda, Maryland. Subjects visit the NIH for initial evaluation and eligibility assessment and at the primary endpoint, with interim follow-up conducted via telehealth and local laboratory assessments.
Subjects must be 18 years of age or and older, diagnosed with DBA and with clinically significant anemia (hemoglobin < 9/dL and/or requiring 2 units PRBC per 8 weeks). They must have relapsed and/or steroid refractory disease or be intolerant of systemic steroids, and be without significant comorbidities. Ongoing transfusions and chelation therapy are allowed, but other DBA-directed therapy, most notably steroids and growth factors, are not.
Each subject will take oral bitopertin daily, escalating the dose monthly (5 mg, 10 mg, 20 mg, 40mg, and 60 mg) until reaching either the study maximum dose of 60 mg, the minimum effective dose (MED), or the maximum tolerated dose (MTD).
Subjects will be followed for 8 months (32 weeks), including a minimum of 4 months (16 weeks) at the final dose (MED, MTD or study maximum). Response will be assessed on an ongoing basis and will be defined as an increase in hemoglobin ≥by 1.5 g/dL and/or a reduction in the units of PRBC transfusions by ≥50% from baseline. Furthermore, a robust response will be defined as a hemoglobin ≥10 g/dL and transfusion independence for 8 weeks. Patients with response (robust or non) by the 8-month primary endpoint will be allowed to continue extended therapy for 3 additional years.
The primary endpoint is erythroid response. Secondary endpoints include safety as defined by the toxicity profile over 8 months of therapy and on extended therapy, relapse, survival, clonal evolution, health-related quality of life, and ancillary laboratory studies including the impact of bitopertin upon erythroid cell dynamics.
The study employs a minmax design. A minimum of 15 patients will be enrolled. If the minimum efficacy threshold is met or surpassed (≥13% response rate), an additional 10 patients will be enrolled.
Status: This study has been granted an FDA IND (165778) and is open for enrollment. It is monitored under the NIH Institutional Review Board (001528-H) and is registered with the U.S. National Library of Medicine (NCT05828108).
Funding: This study is supported by the NHLBI Division of Intramural Research and in part through a Cooperative Research and Development Agreement (CRADA) with Disc Medicine, Inc. (Watertown, MA).
Disclosures
Young:Disc Medicine: Research Funding. Doty:Disc Medicine: Research Funding. Abkowitz:Disc Medicine: Membership on an entity's Board of Directors or advisory committees, Research Funding. Savage:Disc Medicine: Current Employment, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal