Introduction: Immune-mediated graft failure (also termed graft rejection) is a feared complication of hematopoietic stem cell transplant (HSCT) and there is no effective intervention. Multiple pre-transplant risk factors for graft failure are known and recent studies from our groups identified interferon gamma (IFNγ) as a mechanistically important and pharmacologically targetable cytokine involved in graft failure pathophysiology. CXCL9, a downstream marker of IFNγ production, was also identified as a biomarker. We describe an international multicenter retrospective pooled analysis of emapalumab (EMA), an IFNγ neutralizing agent, for the prevention/treatment of immune-mediated graft failure after HSCT.
Methods: The safety and preliminary efficacy of EMA were studied in predominantly pediatric patients who received EMA using two different strategies: 1) graft failure treatment and 2) graft failure prophylaxis. Patients at US centers were treated with EMA if they developed clinical and laboratory signs of graft failure after HSCT. All treatment patients met 2 or more of the following criteria: 1) HLA mismatched donor and/or prior graft failure, 2) unexplained fever >39°C (or >38°C if prior history of graft failure) after HSCT, 3) absolute neutrophil count decline after initial engraftment, 4) delayed neutrophil engraftment after HSCT, 5) real-time CXCL9 > 2.5x upper limit of normal (ULN). Temperature and CXCL9 cutoffs were based on a prior graft failure biomarker publication (Sabulski et al, Blood Advances 2021). Patients treated at European centers received prophylactic EMA to prevent graft failure if they were deemed at high-risk prior to transplant. High-risk patients had a prior history of graft failure after HSCT or had two or more of the following established risk factors: a) affected by a disease with high risk of graft failure, including acquired severe aplastic anemia, thalassemia, primary HLH; b) ex-vivo T cell-depleted stem cell product; c) transplant from unrelated cord blood unit or mismatched related/unrelated donor.
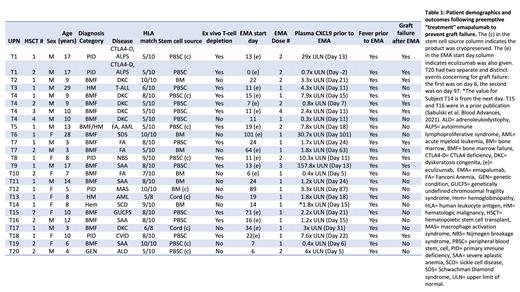
Results: Thirty-six HSCTs used EMA for graft failure treatment or prophylaxis and were included in this study.EMA was used for graft failure treatment in 25 HSCTs wherein patients developed clinical and laboratory signs of graft failure (Table 1). Most HSCTs in the treatment cohort were performed for bone marrow failure syndromes (n=16). All patients tolerated EMA without complications and no side effects or infections were attributed to EMA. Fifty six percent (14/25) of patients treated with EMA for impending graft failure engrafted and had resolution of graft failure concerns, while the remaining 44% suffered graft failure. Responders had a median ANC of 0.29 x10 3 cells/μL (range 0.05-0.79) prior to EMA, which increased to a median of 0.6 x10 3cells/μL (range, 0-5.1) and 2.97 x10 3 cells/μL (range, 0.85-8.7) on days 3 and 7 after EMA dosing. Eighty percent (20/25) of CXCL9 levels were elevated in the treatment cohort prior to EMA dosing. The median CXCL9 level prior to EMA dosing was elevated 2.4 x ULN (range, 0.3-157.8). The median pre-EMA CXCL9 level in patients who ultimately suffered graft failure in the treatment cohort was 3.3x ULN (range, 0.7-157.8) compared to a median of 2.2x ULN (range, 0.3-30.4) in subjects who had signs of graft failure but did not suffer graft failure (p=0.24). Eleven patients received prophylactic EMA based on a prior history of graft failure or other pre-transplant risk factors. EMA was well tolerated in these patients and 91% (10/11) of patients engrafted while one patient with prior history of graft failure suffered recurrent graft failure. The pre-HSCT (day -2) CXCL9 level in the lone prophylactic patient who suffered graft failure was 4x the ULN.
Conclusions: This study reports the largest cohort of HSCTs that used EMA therapy for the novel purpose of treating or preventing graft failure. EMA was well tolerated in all patients. Efficacy conclusions are preliminary and limited by the study design, however our treatment study successfully identified a cohort of patients with a markedly high graft failure rate (44%) and we show preliminary evidence to suggest emaplaumab prevented graft failure in patients who had signs of impending graft failure at the time of treatment. The safety and preliminary efficacy observations in this study support a larger prospective study of IFNγ blockade for graft failure prevention after HSCT.
OffLabel Disclosure:
Sabulski:SOBI: Consultancy. Jodele:SOBI: Consultancy. Myers:Elixirgen Therapeutics: Other: Industry sponsored clinical trial ; Incyte: Other: Clinical trial funding. Merli:MEDAC: Speakers Bureau; SOBI: Consultancy; JAZZ: Consultancy, Honoraria; Amgen: Speakers Bureau.
Emapalumab is an interferon gamma inhibitor approved for use in refractory hemophagocytic lymphohistiocytosis. In this study we investigate the use of emapalumab to prevent immune mediated graft failure after hematopoietic stem cell transplant.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal