Background:
Venous thromboembolism (VTE) and bleeding are common complications amongst allogeneic and autologous hematopoietic stem cell transplant (HSCT) patients. Balancing risk of bleeding and thrombosis in this population is particularly challenging as HSCT patients are concurrently pancytopenic and coagulopathic. However, risk factors for bleeding and thrombosis in HSCT patients are understudied. We aimed to derive a combined bleeding and VTE risk assessment tool to be used in the immediate post-transplant period (90 days).
Methods:
We conducted a retrospective cohort study of adult patients who underwent allogeneic or autologous HSCT between January 1, 2011 and December 31, 2021 at a tertiary care centre in Canada. The risk models patients were followed from transplant until occurrence of the event of interest with last follow up at Day 90 post-transplant, or death. Primary outcome for VTE was the occurrence of a confirmed thrombosis including proximal upper and lower extremity deep vein thrombosis, pulmonary embolism, or thrombosis of unusual sites, including cerebral and splanchnic. Primary outcome for bleeding was the occurrence of a major or clinically significant non-major bleeding event, not related to anticoagulation, as per the definition of the International Society of Thrombosis and Hemostasis. Group characteristics were compared using chi-square, Fisher's exact, or Student's T-tests as appropriate. Potential predictors for VTE and bleed amongst our patients were evaluated using single variable logistic regression and confirmed with multiple variable logistic regression with forward selection. For continuous variables, optimal cut-off points were estimated using ROC curves. Final VTE and bleed risk scores were derived based on weighted variables and compared using Cox regression with non-parametric bootstrapping used for internal validation.
Results:
A total of 476 patients (317 autologous, 159 allogeneic) were included. 47 patients (9.8%) suffered from VTE, and 32 patients (6.7%) suffered from bleed unrelated to anticoagulation within 90 days post-HSCT.
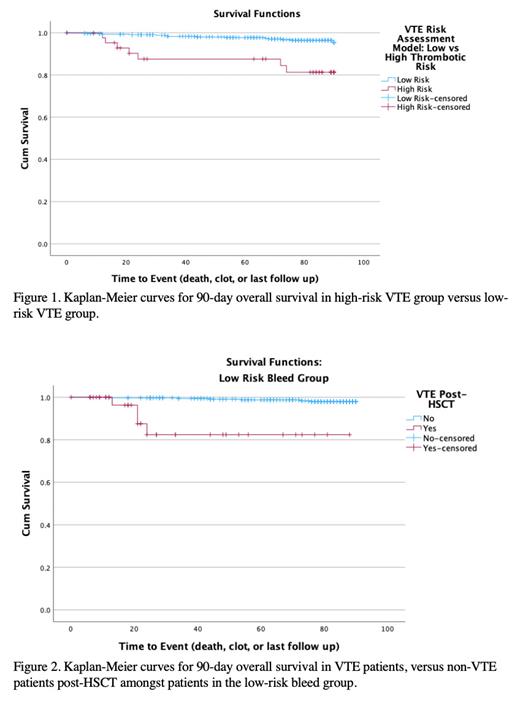
A VTE risk assessment tool was derived, and internally validated, and it included: second central venous catheter insertion (due to complications from first central line) (2 points), and previous cancer treatment with steroids (1 point). The overall cumulative incidence of VTE was 31.8% in the high-risk group (>2 points) versus 7.6% in the low-risk group (0-1 points). The high-risk group was associated with higher mortality at 90 days (15.9% versus 3.2%, p <0.001; Figure 1).
A bleeding risk assessment tool was also derived, and internally validated, and it included: baseline (day 0 of transplant) platelet count of <90 (1 point), and baseline hemoglobin of <96 (1 point). The overall cumulative incidence of bleed was 17.2% in the high-risk group (2 points), versus 4.3% in the low-risk group (0-1 points). The high-risk group was associated with higher mortality at 90 days (12.6% versus 2.6%, p<0.001).
Amongst the low-risk bleed group, overall cumulative incidence of VTE was 10.03%, with 31.03% in the high-risk VTE group versus 8.33% in the low-risk VTE group (p<0.001). The positive VTE group was associated with higher mortality at 90 days (10.26% versus 1.72%, p=0.001; Figure 2). Amongst the high-risk bleed group, overall cumulative incidence of VTE was 9.19%, with 33.33% in the high-risk VTE group, versus 4.17% in the low-risk VTE group (p<0.001). The positive VTE group was associated with higher mortality at 90 days (37.5% versus 10.13%, p=0.026).
Conclusion:
We derived a predictive score, VTE and Bleeding in Marrow Transplant (VBMT) - risk score, for both VTE and bleeding in the immediate post-transplant period (90 days), to be used in conjunction with other criteria for bleeding and thrombosis. Amongst the low-risk bleed patients, we recommend strong consideration of chemical VTE prophylaxis, especially amongst the high-risk VTE group. These risk assessment models will help stratify patients based on bleed and thrombosis risk, and ultimately help guide VTE prophylaxis and surveillance strategies amongst HSCT patients.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal