Abstract
Introduction
Paroxysmal nocturnal hemoglobinuria (PNH) clones are characterized by the absence of cell surface glycosylphosphatidylinositol (GPI)-anchors owing to somatic mutations in the PIGA gene on the X chromosome. Large PNH clones derived from PIGA mutant multipotent progenitors (MPPs) result in clinically significant hemolysis. In immune aplastic anemia (AA), the PNH clone represents immune escape from T cell-mediated pathogenesis and is usually small (<1%), sometimes likely derived from lineage-committed progenitors (LCPs), with detectable GPI-anchor deficient (PNH-type) cell populations only in granulocytes and monocytes. Small PNH-type cells are also present in healthy individuals at a much lower frequency (<0.003%) than in AA patients. The nature of the small PNH-type cells is not fully understood because of the difficulties in analyzing rare cell populations.
Methods
In this study, we developed a method to detect PIGA mutations within a small population of cells at single-cell resolution. PNH-type cells were enriched from peripheral blood leukocytes using magnetic microbeads and monoclonal antibodies specific for GPI-anchored proteins. The enriched cell fraction was then subjected to fluorescence-activated cell sorting after staining with lineage-specific markers and fluorescein-labelled proaerolysin. We sorted a limited number (100 cells in males and 50 cells in females) of PNH-type granulocytes (PNH-Gs) and T cells (PNH-Ts) as well as wild-type cells into separate tubes, and their PIGA genes were directly amplified by multiplex PCR without a DNA extraction procedure for subsequent deep nucleotide sequencing. To ensure the specificity of PIGA mutation detection, we set cut-off thresholds of 2% for single-nucleotide substitutions and 1% for indels, in addition to the thresholds based on base-position-specific error rates in 10 sets of 2000 wild-type cells, as previously reported (Zaimoku et al. Blood 2021).
Results
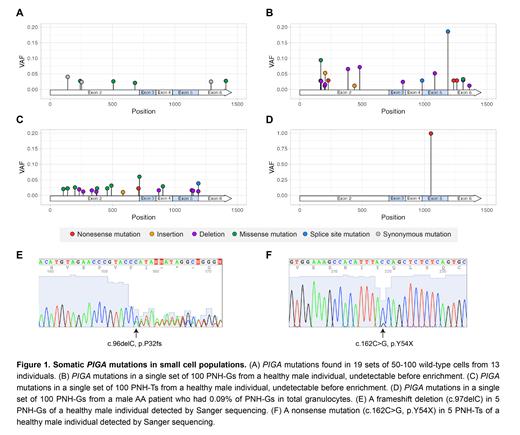
In 19 sets of 50-100 wild-type cells from 13 individuals, we detected only 7 mutations with variant allele frequencies (VAFs) ranging from 2.1% to 4.2% (Figure 1). Three of these were synonymous mutations, and the remaining 4 were missense mutations, suggesting that these mutations may be true passenger mutations rather than sequencing errors. In all 6 healthy individuals, small PNH-type cells exhibited a polyclonal pattern. We detected numerous small, likely inactivating, PIGA mutations in PNH-Gs, with a median of 11 (range, 6-18) mutations per 100 cells. In 3 individuals assessed twice, approximately half of the PIGA mutations were detected in both sets of PNH-Gs, whereas the remaining mutations were unique to each set. Similarly, PNH-Ts from 4 healthy individuals showed polyclonality, with a median of 14 (range, 6-22) mutations per 100 cells. Interestingly, PIGA mutations were not shared between PNH-Gs and PNH-Ts, except for 2 splice site mutations with VAFs <10%. The polyclonal pattern was further confirmed by Sanger sequencing, which successfully detected 7 unique PIGA mutations in 5 sets of 5 PNH-type cells (instead of 100 cells) in a healthy donor, whereas no mutations were detected in 3 sets of 5 wild-type cells, except for 1 synonymous mutation. In contrast, small PNH-Gs of 5 AA patients, with frequencies of 0.02%, 0.08%, 0.09%, 0.2%, and 0.5% of total granulocytes, were primarily derived from single PIGA mutant clones, which accounted for 90%-99% of PNH-G populations. In another patient with AA, PNH-Gs with a frequency of 3.3% were derived from 2 dominant clones. Notably, the dominant PIGA mutations in the PNH-Gs of AA patients were all detectable in PNH-Ts, even in 2 patients whose PNH-Ts were not detectable (<0.003%) and in 3 patients whose percentages of PNH-Ts were 0.004%, 0.02%, and 0.08% before microbead-based enrichment.
Discussion
Our novel sequencing approach provides insight into the clonality and lineage distribution of small PNH-type cells without in vitro culture manipulation. In healthy individuals, most PNH clones originate from LCPs, where many cell divisions occur, thereby providing opportunities for the acquisition of PIGA mutations. In immune AA, only PIGA mutations acquired at the MPP level lead to clonal proliferation, strongly suggesting that the immune pathogenesis of AA targets MPPs, rather than LCPs. A clonality analysis of small PNH-type cells thus helps diagnose the immune pathogenesis of AA.
Disclosures
Zaimoku:Nippon Shinyaku: Research Funding. Yamazaki:Kyowa Kirin: Honoraria; Novartis Pharma: Honoraria. Miyamoto:Otsuka Pharmaceutical: Honoraria; MSD: Honoraria; Daiichi Sankyo: Honoraria; Novartis: Honoraria; Bristol: Honoraria; Janssen: Honoraria; Astellas: Honoraria; Kyowa Kirin: Honoraria, Research Funding; AbbVie: Honoraria; Amgen: Honoraria; Chugai Pharmaceutical: Research Funding. Nakao:Novartis Pharma: Honoraria; Kyowa Kirin: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal