Tp53 mutated acute myeloid leukemia (AML) is a highly challenging subtype to treat due to its aggressive nature and resistance to standard therapies. Even in cases where initial responses to conventional treatments occur, residual minor clonal populations harboring p53 mutations place patients at a higher risk of relapse and lower overall survival rates. Thus, innovative treatment strategies are essential to tackle the challenges of p53 mutated AML and enhance patient outcomes.
Although it plays a substantial role in G1/S and G2/M checkpoints, p53's significance in the intra-S-phase checkpoint is limited. This checkpoint is vital in detecting replication defects and its disruption leads to catastrophic genome instability, massive DNA breakage, and cell death. We endeavored to target this pathway by employing a non-ATP cell cycle inhibitor, LBS-007, that blocks the kinase activity of CDC7 that is responsible for phosphorylating MCM2 prior to DNA replication initiation. Thus, we hypothesized that inhibition of CDC7 can disrupt DNA synthesis, leading to genomic instability and potentially p53-independent cell death.
Initially, we explored LBS-007 as a single agent in a p53 deleted (17p-) AML cell line and two AML lines with hot-spot p53 mutation (R175H or R248Q). LBS-007 showed significant effectiveness in all three cases with altered p53 status and with GI 50 values comparable to those observed in wild-type p53 AML cells (62 nM, 40 nM, and 59 nM versus 133 nM). To further enhance its therapeutic impact, we investigated combinations with other DNA replication inhibitors. When used sequentially with LBS-007, hydroxyurea provided a robust response yielding GI 50's between 1 and 3 nM for LBS-007, effectively blocking DNA replication origins (LBS-007) and inducing fork stalls (HU). Surprisingly, the combination of LBS-007 and an ATR inhibitor, Ceralasertib, had a paradoxical effect, suggesting the effectiveness of LBS-007 requires intra-S-phase checkpoint activation.
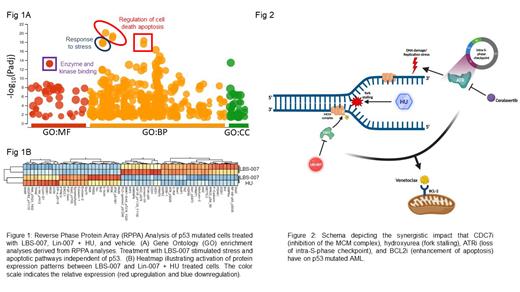
To determine the underlying molecular mechanisms, we conducted RPPA analyses on p53 defective AML cells treated with LBS-007. We observed significant upregulation of apoptotic proteins and pathways when used as a single agent (Figure 1A). However, in combination with hydroxyurea, activation of DNA damage sensors and intra-S-phase checkpoint proteins were overrepresented (Figure 1B). The observed effectiveness of LBS-007 in driving robust p53-independent apoptosis and cell cycle checkpoints indicates its potential as a powerful new agent to treat p53-mutated AML.
To discover synergistic combinations that enhance the efficacy of LBS-007, we utilized venetoclax, a BCL2 inhibitor that facilitates apoptosis. The inclusion of venetoclax significantly increased the efficacy of LBS-007, resulting in combinatorial GI 50's below 10 nM in both p53 mutated and p53 deleted AML. Most impressively, the combination of LBS-007 and venetoclax was exponentially enhanced when used in conjunction with ceralasertib (4.8 pM). Collectively, these results provide compelling evidence that combining CDC7 inhibition and venetoclax with a potential ATR inhibitor will have a profound impact on treatment of p53 altered AML (Figure 2).
We are now evaluating the efficacy of these combinations using xenograft transplant models. To this end, cohorts of mice transplanted with AML cell lines harboring either wild-type or mutated p53 will be treated with strategies identified through our in vitro and RPPA analyses. Further studies will address the cooperation between mutations common to AML and p53 alterations using defined PDX models.
Our results identify a unique and potentially efficacious strategy to treat one of the most difficult to treat subtypes of AML. These findings will provide the rationale for potential clinical trials using CDC7 inhibition in p53 mutated or R/R AML.
Disclosures
Post:LinBioscience: Research Funding. Wang:LinBioscience: Current Employment. Chang:LinBioscience: Current Employment. Frattini:Cellectis, Inc.: Current Employment, Current equity holder in publicly-traded company; Lin Biosciences: Consultancy. Maiti:Lin BioScience: Research Funding; Celgene: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal