Background
Ph-like acute lymphoblastic leukemia (ALL) is high risk and comprised of many kinase activating gene fusions including those involving the ABL1 gene ( ABL1-rearranged, ABL1r). ABL1r ALL is associated with poor relapse-free and overall survival and incidence increases with age. Tyrosine kinase inhibitors (TKIs) such as dasatinib effectively target BCR::ABL1+ ALL and are in clinical trials for ABL1r ALL. However, resistance to TKIs is often observed, and novel treatments are required. The allosteric inhibitor asciminib binds the myristate pocket of Bcr-Abl kinase and is currently in Phase III trials for treatment of BCR::ABL1+leukemias. We assessed asciminib efficacy in ABL1r ALL.
Methods
Ba/F3 cells were transduced with four ABL1 fusions observed in ALL and asciminib efficacy evaluated in AnnexinV/7-AAD cell death assays (LD 50). Specific regions of the ABL1 fusion partner were deleted by site-directed mutagenesis (SDM). STAT5/CRKL phosphorylation and hCD45 levels were investigated by flow cytometry. Cells from three NUP214::ABL1 ALL patients were injected into NSG mice to establish patient-derived xenograft (PDX) models. Once mice reached 5% hCD45+ cells in peripheral blood (PB), treatment was commenced: vehicle control, dasatinib, asciminib. At experimental endpoint (>50% hCD45+ cells in PB) mice were humanely killed and organs harvested for analysis.
Results
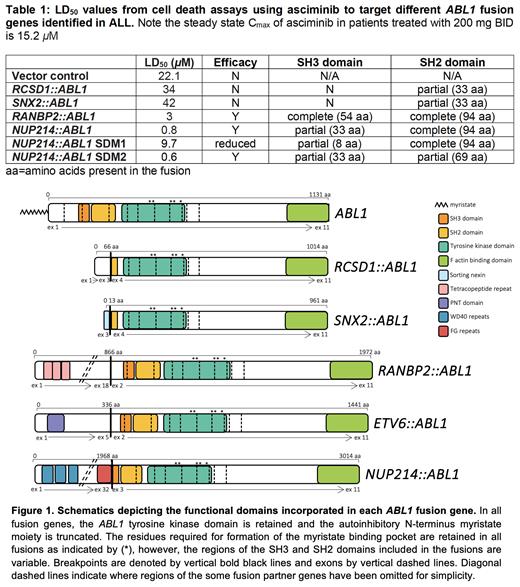
Transduced Ba/F3 cells demonstrated varying sensitivity to asciminib (Table 1). While asciminib was not efficacious against Ba/F3 RCSD1::ABL1 and SNX2::ABL1 cells, Ba/F3 RANBP2::ABL1 (Heatley et al 2021)and NUP214::ABL1 cells demonstrated LD 50 concentrations within the clinically achievable range (3 µM, p<0.0001; 0.8 µM, p=0.0238 compared with control, respectively). Following asciminib treatment, Ba/F3 NUP214::ABL1 cells demonstrated reduced phosphorylation of STAT5 (mean fluorescence intensity [MFI]=1820 to 790, 57% reduction) and CRKL (MFI=2069 to 951, 54% reduction), confirming asciminib inhibits kinase signalling. No phosphorylation of STAT5 or CRKL was observed in control cells (MFI=290 and 406, STAT5 and CRKL, respectively). Additionally, ex vivo assessment of ETV6::ABL1 patient blasts demonstrated reduced phosphorylation of STAT5 (MFI=3395 to 1788, 47% reduction) and CRKL (MFI=5039 to 3000, 40% reduction) following asciminib treatment.
Interrogation of the different ABL1 fusion breakpoints (Figure 1) revealed fusions demonstrating asciminib sensitivity retained exons 2 or 3 of ABL1 (corresponding to complete/partial SH3 and complete SH2 domains). Conversely, gene fusions lacking exons 1-3 (truncating the SH3 domain and retaining a partial SH2 domain) were resistant to asciminib suggesting a critical region in the SH3 and/or SH2 domain/s required for asciminib efficacy. To further investigate this we systematically deleted regions of ABL1 exon 3 using the NUP214::ABL1 construct as the model system and generated transduced Ba/F3 cells expressing different SDM regions. Cell death assays indicated the SH3 domain is critical for asciminib efficacy (Table 1).
Asciminib also demonstrated efficacy in pre-clinical mouse modelsof NUP214::ABL1 ALL (where ABL1 exon 3 and partial SH3 domain is retained). Asciminib treatment significantly increased survival outcomes compared with control mice (84 vs 46d, p=0.01) by a time comparable to that of dasatinib treatment (93 vs 46d, p=0.0046). Results were confirmed in two additional NUP214::ABL1 PDX models demonstrating asciminib's potential to treat NUP214::ABL1 patients. Asciminib treatment reduced spleen and liver weights to that of healthy mice and resolved the leukemic immunophenotype of PB and bone marrow cells.
Conclusion
ABL1r ALL is associated with high rates of treatment failure and relapse. For the first time, we demonstrate the efficacy of asciminib in this high risk disease. Our data support addition of asciminib to treatment regimens of patients with NUP214::ABL1 ALL, the most common ABL class gene fusion affecting up to 6% of B-ALL patients and up to 10% of T-ALL patients. We also identify a critical region of ABL1 required for asciminib efficacy in ABL1r ALL. We propose interrogation of ABL1r patient breakpoints to determine inclusion/exclusion of the ABL1 SH3 domain as a surrogate test to be used in conjunction with in vitro kinase sensitivity assays for predicting whether a patient is likely to respond to asciminib treatment.
Disclosures
Yeung:Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding. Hughes:Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Terns Pharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Enliven: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal