Introduction: Brentuximab vedotin (BV) is one of the most active systemic agents for treating mycosis fungoides (MF); however, treatment duration and the duration of response is frequently limited by the development of peripheral neuropathy (PN). In the phase III ALCANZA study of BV, 44/66 (67%) of patients (pts) treated with BV developed PN, including grade 2 (G2) in 32% and grade 3 (G3) in 10%. The overall response rate (ORR) to BV was 65% and median duration of response (DOR) was 15.1 months (mo) (Prince et al, Lancet 2017). We hypothesized that reduced-dose BV would improve tolerability without compromising efficacy and therefore facilitate a longer duration of response for pts with MF.
Methods: This is a multicenter phase II study evaluating reduced-dose BV in pts with relapsed or refractory MF who are BV-naive. Cohort 1A evaluated BV at 0.9 mg/kg every 3 weeks and cohort 1B evaluated BV at 1.2 mg/kg every 3 weeks. For each dose level, we utilized a Simon two-stage minimax design in which we set the desired ORR at 65% (as reported in ALCANZA) and the null hypothesis at 35%. Accordingly, stage I required 4 responses among the first 9 pts enrolled to expand to a total of 19 pts. In stage II, 11 responses were required to declare the dose promising.
Global response was evaluated every 4 cycles using the International Society for Cutaneous Lymphomas consensus criteria (Olsen et al, JCO 2011). PN was evaluated according to the NCI Common Terminology Criteria for Adverse Events v. 4.0.3 and Total Neuropathy Score (TNSc). Additional outcomes include Health Related Quality of Life (HRQoL) measured by the FACT-Lym, FACT-GOG_NTX and Skindex-29 questionnaires completed by pts every four cycles.
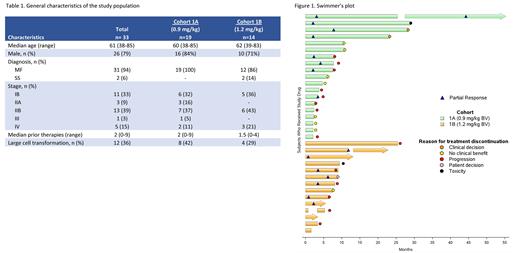
Results: Among 19 pts enrolled on cohort 1A, median age was 60 years (yrs) (range, 38-85) and 16/19 (84%) were male. Majority had stage I/II disease (n = 16, 84%) and 42% of pts had large cell transformation. Median number of prior therapies was 2 (range 0-9) (Table 1). 8/19 pts (42%) experienced PN, including 3/19 (16%) G2 and 0/19 ≥ G3. ORR was 42% (8/19) (8 PR, 0 CR). Median DOR was 19.6 mo (95% CI, 5.9 ‐ NR) (Figure 1).
The primary efficacy endpoint for cohort 1A was not met, leading to enrollment onto cohort 1B. To date, 14 of planned 19 pts enrolled onto cohort 1B. Majority had MF (86%), median age was 62 yrs (range 39-83) and 10/14 (71%) were male. 11/14 (79%) were early stage and 4/14 (29%) had large cell transformation. Median number of prior therapies was 1.5 (range 0-4). 10/14 (71%) pts experienced PN, including 4/10 (40%) G2 and 0/10 ≥ G3. The ORR is 50% (7 PR, 0 CR) and the median DOR has not been reached (95% CI, 5.4 ‐ NR).
Across the 2 cohorts, additional BV-related adverse events seen in >10% of pts included infusion-related reactions (G1, n = 3; G2, n = 1), hepatic transaminase elevation (G1, n = 4; G2, n = 1), diarrhea (G1, n = 4; G3, n =1), nausea (G1, n = 4; G2, n =1), fatigue (G1, n = 5; G2, n = 2), and maculopapular rash (G1, n = 4; G2, n = 3; G3, n =2).
Conclusion: Treatment of MF with reduced-dose BV of 0.9 mg/kg was associated with lower clinical activity (ORR 42%) than full-dose BV (1.8 mg/kg, ORR 65%). However, while recognizing the limitations of cross-trial comparisons, there were numerically lower rates of PN and a longer median DOR than with full dose BV on the ALCANZA trial. Although unable to reject the null hypothesis for cohort 1A, 0.9 mg/kg dosing allowed for prolonged disease control and diminished toxicity for responding pts. As MF is a chronic, typically incurable condition, prolonged duration of treatment is particularly important. Enrollment onto cohort 1B (1.2 mg/kg) is ongoing; we will present updated results along with pt reported outcomes at the conference.
Disclosures
Geller:RAFA Laboratories Ltd: Other: Professional services and activities; Takeda Pharmaceuticals: Other: Professional services and activities; UptoDate: Other: Intellectual Property Rights. Ghione:AstraZeneca Pharmaceuticals: Consultancy; Kyowa Hakko Kirin: Consultancy; Secura Bio: Consultancy; Kite, A Gilead Company: Research Funding. Epstein-Peterson:OncLive: Honoraria; Amgen: Research Funding; WebMD: Honoraria; Kymera: Research Funding; Viracta: Research Funding. Khodadoust:Nutcracker Theraputics: Research Funding; Daiichi Sankyo: Membership on an entity's Board of Directors or advisory committees; CRISPR Theraputics: Research Funding. Kim:CRISPR Therapeutics: Research Funding; Elorac: Research Funding; Trillium: Research Funding; Corvus: Research Funding; Innate: Research Funding; Kyowa Kirin: Research Funding; Citius: Research Funding; Eisai: Research Funding; Takeda: Research Funding; Drenbio: Research Funding. Horwitz:Auxilius Pharma: Consultancy; Crispr Therapeutics: Research Funding; Shoreline Biosciences, Inc.: Consultancy; Affimed: Research Funding; ONO Pharmaceuticals: Consultancy; Trillium Therapeutics: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; SecuraBio: Consultancy; Kyowa Hakko Kirin: Consultancy, Research Funding; ADC Therapeutics: Research Funding; Tubulis: Consultancy; Yingli Pharma Limited: Consultancy; Millenium: Research Funding; Seattle Genetics: Research Funding; Verastem/SecuraBio: Research Funding; Cimieo Therapeutics: Consultancy; Takeda: Consultancy, Research Funding; Abcuro Inc.: Consultancy; Celgene: Research Funding. Moskowitz:Beigene: Research Funding; ADC Therapeutics: Research Funding; Seattle Genetics: Honoraria, Research Funding; Merck: Honoraria, Research Funding; Bristol-Myers Squibb: Research Funding; Incyte: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal