The clinical course of patients with advanced forms of myelofibrosis (MF) can frequently be punctuated by thrombocytopenia. Marrow biopsies from these MF patients uniformly reveal megakaryocytic (MK) hyperplasia and dysplasia irrespective of the patient's platelet count.
We have performed studies to better understand the origins of MF associated thrombocytopenia. We first examined the expression of G6b-B (MK-lineage specific immunoreceptor tyrosine-based inhibition motif) which is essential in promoting MK maturation, normal platelet release, platelet size and function (Becker. Blood Adv 2022). Recent reports have indicated that genetic deletion in G6b-B and loss-of-function mutations in both murine models and man lead to severe macro-thrombocytopenia and increased bone marrow fibrosis (Becker. Blood Adv 2022; Hofmann. Blood 2018; Melhem. Eur J Hematol 2017), indicating a possible role for this receptor in the development of thrombocytopenia in MF patients.
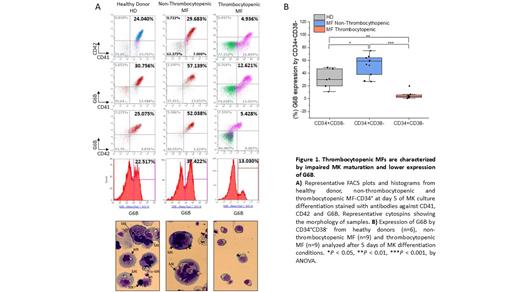
We generated populations of MKs from mononuclear cells or CD34 + from thrombocytopenic or non-thrombocytopenic MFs and healthy donors (HDs) using a two-step culture system as previously described (Mosoyan. Leukemia 2017). A progressive increase of MK maturation (CD41 +CD42 +) was observed in HDs (28.5%±7) and non-thrombocytopenic MFs (32%±13.6) with a concomitant increase of total G6B expression 27%±9 and 38%±14 respectively. By contrast, MKs generated from thrombocytopenic MFs patient cells had limited CD41 +CD42 + (7%±5) and G6B (8.7%±4.7) expression (p<0.05). In addition, morphological analysis revealed that MKs from thrombocytopenic MF patients were small with hypolobulated nuclei while the MKs generated from HDs and non-thrombocytopenic MFs were larger and contained greater numbers of lobes ( Figure 1A). Psaila and coworkers have previously reported that MF is characterized by MK biased hematopoietic stem cells (HSCs) identified by increased expression of G6B (Psaila. Mol Cell 2020). We, however, determined that a reduced proportion of CD34 +CD38 - HSCs from thrombocytopenic patients expressed G6B as compared to non-thrombocytopenic MF or HD CD34 +CD38 - cells (p<0.05) ( Figure 1B). These studies indicate a reduced proportion of MK biased HSCs in thrombocytopenic MFs which likely leads to impaired MKs maturation.
We next explored if the MF associated pro-inflammatory milieu also contributes to the MF MK maturation block. Thrombocytopenic MF plasma contained higher levels of YKL-40 (chitinase-3-like-1 protein), TNFα and TPO than HD plasma. For instance, MF plasma was characterized by higher levels of YKL-40 (median141,321 pg/mL) in comparison to HDs (median 19,380 pg/mL). Furthermore, YKL-40 levels were significantly higher in thrombocytopenic (median 187,696 pg/mL; p<0.05) than non-thrombocytopenic (median 78,082 pg/mL) MFs. In addition, TNFα and TPO plasma levels were also significantly higher in plasma from thrombocytopenic than non-thrombocytopenic MFs (p<0.05). We also observed that addition of either rYKL40 or rTNFα to HD-MK cultures resulted in delayed MK maturation as assessed by a reduced proportion of cells expressing CD41 +CD42 + (from 30% to 16.5% and 6.5% respectively) and G6B (from 35.5% to 22.5% and 9.5% respectively).
We have previously reported that the treatment of MFs with a protein trap for TGFβ1 led to increases in platelet numbers (Mascarenhas. Clin Cancer Res 2023). The plasma from these patients prior to treatment not only had elevated levels of TGFβ1 (median 6433,917 pg/mL) but also YKL40 (median 275,640 pg/mL). Treatment of the patients with the TGFβ protein trap reduced both TGFβ1 (median 608,087 pg/mL) and YKL-40 (median 160,733 pg/mL) plasma levels, suggesting a regulatory loop between YKL-40 and TGFβ. We further explored this loop by treating the JAK2V617F+ HEL cell line with rYKL-40 in combination with rTGFβ1, which resulted in greater expression of YKL-40 (1.6 FI) than treated cells with rYKL-40 alone. In addition, treatment of HEL cells with a pan TGFβ inhibitor reduced YKL-40 protein expression (1.55 to 0.38). These data suggest the existence of an interplay between YKL40 and TGFβ on MF-MK maturation.
Overall, these experiments indicate that thrombocytopenia in MF patients is due to intrinsic abnormalities in G6B originating at the level of the HSC compartment resulting in impaired MK maturation which can be further enhanced by the actions of a number of pro-inflammatory cytokines.
Disclosures
Mascarenhas:Bristol Myers Squibb, Celgene, CTI BioPharma, Geron, Incyte Corporation, Janssen, Kartos Therapeutics, Merck, Novartis, PharmaEssentia, Roche; Participated in consulting or advisory committees - AbbVie, Bristol Myers Squibb, Celgene, Constellation Pharmac: Research Funding; Incyte, Novartis, Roche, Geron, GSK, Celgene/BMS, Kartos, AbbVie, Karyopharm, PharmaEssentia, Galecto, Imago, Sierra Oncology, Pfizer, MorphoSys, CTI Bio: Consultancy; Bristol Myers Squibb, Celgene, Constellation Pharmaceuticals/MorphoSys, CTI BioPharma, Galecto, Geron, GSK, Incyte Corporation, Karyopharm Therapeutics, Novartis, PharmaEssentia, Prelude Therapeutics, Pfizer, Merck, Roche, AbbVie, Kartos: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie, Bristol Myers Squibb, Celgene, CTI BioPharma, Geron, Incyte Corporation, Novartis, Janssen, Kartos Therapeutics, Merck, PharmaEssentia, Roche: Research Funding; GSK: Honoraria; AbbVie, CTI BioPharma Corp, a Sobi company, Geron, GlaxoSmithKline, Imago, Incyte, Kartos, Kayropharm, MorphoSys, Novartis, Pfizer, PharmaEssentia, Sierra: Consultancy. Hoffman:Silence Therapeutics: Consultancy; Kartos Abbvie: Research Funding; Curis: Research Funding; Karyopharm: Research Funding; Dexcel Pharma: Research Funding; TD2: Research Funding; Summitomo: Research Funding; Dompe: Patents & Royalties; Cellinkos: Consultancy; Protagonist Therapeutics: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal