Introduction: JAK inhibitors (JAKi) marked a revolutionary breakthrough in the treatment of patients (pts) with myelofibrosis (MF). The BCL-2 family of proteins have been implicated in both disease development and mechanisms of response/resistance to JAKi. This is further reinforced by preliminary evidence of therapeutic efficacy of combining JAK2 and BCL-xL inhibition.
Aims&Methods: In the current study, we investigated the expression of three major BCL-2 family members in MF pts treated with ruxolitinib (RUX) at baseline and during treatment. Gene expression of BCL-2, BCL2L1 (encoding BCL-xL) and MCL-1 was analyzed by qPCR normalized to endogenous 18S gene using cDNA obtained from peripheral blood (PB) granulocytes; the relative gene expression (expressed as fold-change, FC) was calculated with reference to healthy controls using the Ct (2−ΔΔCt) method.
Results: The study included 19 pts (10 males; median age 61 years) with MF (10 primary, 9 secondary) who received treatment with RUX. Sixteen pts were JAK2-mutated, 2 CALR-mutated, and 1 MPL-mutated. Treatment response was fully evaluable across the entire treatment period. After a median treatment time of 67 (range, 6-121) months, 7 (37%) pts achieved a spleen response (defined as a >50% reduction in palpable splenic length lasting >6 months) and were considered as responders, as compared to 12 (63%) non-responders. We did not find any significant correlation of baseline BCL-2/ BCL2L1/ MCL-1 expression with clinical and molecular characteristics, the only exception being the association between EZH2 mutation and higher BCL2L1 expression.
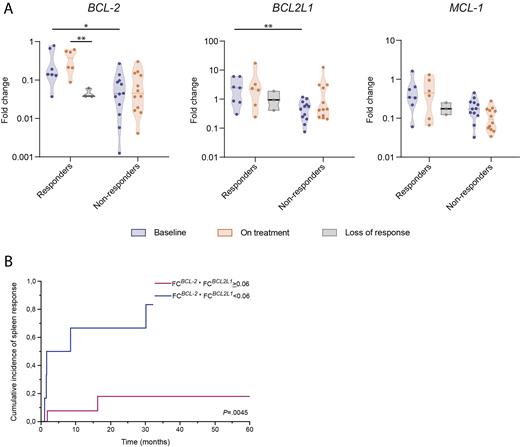
First, we ascertained whether BCL2 family protein expression levels at baseline predicted response to RUX. We found that BCL2 and BCL2L1 were significantly more expressed in responders compared to non-responders, with median FC values of 0.14 vs 0.04 ( P=.0130) and 2.46 vs 0.51 ( P=.0096), respectively (Fig. 1A). A similar, not statistically significant, trend was observed for MCL-1 expression. Accordingly, in univariate regression logistic analysis, both BCL2 and BCL2L1 FCs correlated significantly with the probability of response to RUX.
Then, we incorporated BCL-2 and BCL2L1 gene expression at baseline into a combinatorial score (CS) resulting from the product of the respective FCs (FC BCL-2 * FC BCL-xL). The CS outperformed the individual BCL2 family proteins expression in predicting RUX response, as confirmed in regression logistic analysis. ROC analysis identified 0.06 as the optimal cut-off value of the CS. In univariate analysis, pts with a baseline CS >0.06 had a significantly higher probability of responding to RUX (OR 27.5, 95% CI 2.1-236.7; P=.0037), with a superior cumulative incidence of RUX response at 24 weeks (50% vs 9%, respectively; P=.0045) (Fig. 1B).
Finally, we prospectively assessed the changes in gene expression during treatment with RUX. Six out of seven responder pts had an available PB sample at the time of best response, while all non-responders were evaluated at some point during RUX therapy. Among responders, 5 lost response after a median time of 34 months, and 3/5 had an available sample for analyses at the time of response loss. Although we did not find any significant difference comparing gene expression at baseline and on treatment, we observed a trend for BCL-2 upregulation in responders at the time of best response. Furthermore, non-responders showed a down-regulation of MCL-1 expression during treatment ( P=.05). By comparing gene expression of responder pts at the time of best vs loss of response, we discovered a down-regulation for all the three genes, with a statistical significance that was reached only for BCL-2 ( P=.0471).
Conclusion: Despite study limitations (mainly the small cohort), this preliminary data suggests that the expression levels of BCL2 family proteins may represent a predictor of response to RUX. Specifically, we developed a simple, combinatorial score includingbaseline BCL-2 and BCL2L1 expression that accurately discriminated pts who responded to RUX. Moreover, we showed that BCL2 family protein expression changes during RUX treatment, with potential correlations with both response achievement and loss. Further studies are needed in order to confirm and expand our data.
Disclosures
Vannucchi:Incyte: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Imago BioSciences, Inc., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA: Research Funding; GSK: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Blueprint: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AOP: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Guglielmelli:Abbvie: Other: Other member of advisory board, speaker at meeting, Speakers Bureau; GSK: Speakers Bureau; Novartis: Other: Other member of advisory board, speaker at meeting, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal