Introduction: Bruton tyrosine kinase (BTK) signaling is critical for the proliferation and survival of malignant B lymphocytes in chronic lymphocytic leukemia (CLL). BTK inhibitors (BTKi) are used in the front-line setting as well as for relapsed/refractory (R/R) CLL. Three BTKi are currently approved by the United States Food and Drug Administration (US FDA) for CLL - ibrutinib, acalabrutinib, and zanubrutinib. However, there are some uncertainties on the risk of infections, especially pneumonia, from BTKi due to its potential off-target effects and its immunomodulatory effects on innate and adaptive immune response. The newer second-generation BTKi, acalabrutinib and zanubrutinib, have attenuated off-target effects compared to ibrutinib. We hereby evaluate the incidence of pneumonia, including Pneumocystis jirovecii pneumonia (PJP) and other fungal pneumonia, from BTKi monotherapy in patients with treatment naïve as well as relapsed CLL and compare the incidence among different BTKi.
Methods: This systematic review identified clinical trials from MEDLINE, Embase, and CENTRAL databases from the inception to 30 June 2023. The number of cases with any grade and at least grade 3 lung infection/pneumonia, PJP and other fungal pneumonia, along with the total number of patients in the arms with BTKi monotherapy were extracted from each trial per the Common Terminology Criteria for Adverse Events (CTCAE) v5.0. The incidence meta-analysis was performed using “meta” package version 6.5-0 of R version 4.2.2, via random-effects model. Pooled incidence and its associated 95% confidence interval (CI) were reported for each outcome .I² statistic was used to determine the heterogeneity of the studies. Subgroup analyses by type of BTKi (ibrutinib, acalabrutinib, and zanubrutinib) and treatment history (treatment-naïve and R/R) were performed.
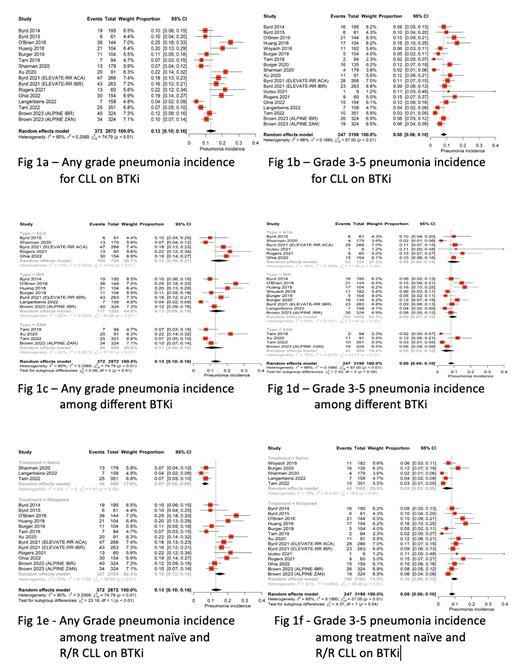
Results: After two rounds of review, eighteen clinical trials containing twenty arms of BTKi monotherapy were eligible for the meta-analysis. Among these, four trials reported the incidences of PJP and three reported on fungal pneumonia. The pooled incidence of any grade pneumonia in patients with CLL on BTKi therapy was 0.13 (95% CI, 0.10-0.16, I2 = 80%) [Figure (Fig) 1a]. The pooled incidence of grade 3 or higher pneumonia was 0.08 (95% CI, 0.06-0.10, I2 = 68%) [Fig 1b]. There were no differences in the incidences of any grade (p = 0.61) or grade 3 and higher pneumonia (p = 0.30) among patients treated with ibrutinib, acalabrutinib, or zanubrutinib in the subgroup analyses [Fig 1c and 1d respectively]. However, the pooled incidences of any grade and grade 3 or higher pneumonia were greater in R/R CLL patients compared to those who were treatment-naïve (0.15 vs 0.07, p<0.01 and 0.10 vs 0.05, p = 0.04, respectively) [Fig 1e and 1f respectively]. The pooled incidences of PJP and other fungal pneumonia were 0.01 (95% CI, 0.00-0.02, I2 = 10%) and 0.01 (95% CI, 0.00-0.02, I2 = 0%), respectively.
Conclusion: Our study showed no significant differences in the incidence of pneumonia of any grade or at least grade 3 among patients treated with newer second-generation BTKi vs first-generation BTKi. Risk of pneumonia should not be a factor to choose among BTKi. Of note, the incidence of pneumonia was higher in R/R CLL patients on BTKi therapy when compared to treatment naïve CLL. R/R CLL are likely to have increased infections due to complement dysfunction and progressive immunodeficiency (decreased IgG and IgA levels). Fungal pneumonia, including PJP, are uncommon in CLL, and the subgroup analyses were not able to distinguish any differences among different BTKi. PJP prophylaxis is still considered during BTKi therapy in treatment naïve and R/R CLL, but true risk vs benefit is unknown.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal