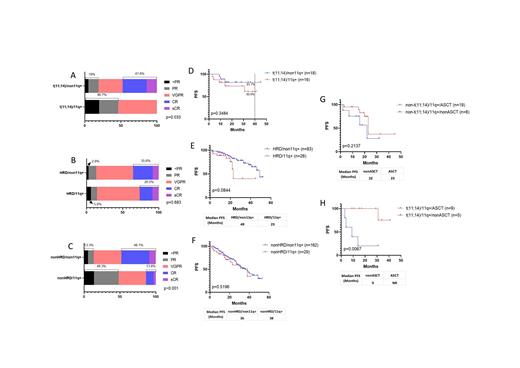
t(11;14) is currently classified as a standard-risk genetic marker for newly diagnosed multiple myeloma (NDMM) patients. But some recent studies have suggested that t(11;14) may be an intermediate-risk marker in multiple myleoma (MM). The clinical significance of t(11;14) in MM is still controversial. Here, using high-resolution single nucleotide polymorphism-based mapping array (SNP-array) analysis and fluorescence in situ hybridization (FISH), the most common gained region of chromosome 11 was identified in 96/611 (15.7%) NDMM patients which located at 11q13.3-q25 (65.7Mb in size and contains 622 genes including CCND1). It demonstrated a statistically significant coexisting with t (11;14) (p<0.001), complex karyotypes (involves at least 6 chromosomes) (p<0.001), high plasma cell ratio in bone marrow (p=0.001) and poor response to VRD (bortezomib, lenalidomide, dexamethasone) treatment (p<0.001), but mutually exclusive with monosome 13, del(13q14), dup1q21 and t (4;14). When exploring the correlation of t(11;14) with genomic copy number variations (CNAs), it was also indicated that t(11;14) was most closely related to 11q gain (39%), while negatively correlated with others including gain of 1q and chromosome 3, 5, 6, 7, 9, 11, 15, 19, loss of 1p, 12p, 14q and chromosome 13 and 22.
With a median follow up time of 22 months, survival analysis of 40 t(11;14) patients, 123 hyperdiploid (HRD) patients showed that 11q gain could have an adverse impact on the progression-free survival (PFS) in both groups. In t(11;14) subgroup, the median PFS (mPFS) were not reached, but PFS at 40 months were 60.9% vs 81.7% with 11q gain vs non-11q gain patients. In HRD subgroup, the mPFS were 23 months vs 49 months with 11q gain vs non-11q gain groups. Autologous hematopoietic stem cell transplantation (ASCT) can significantly improve the PFS of patients with t(11;14) and 11q gain (mPFS for transplant-eligible (TE) vs transplant-ineligible (TIE) patients were not reached vs 9 months, P=0.0067), but not helpful to 11q gain patients without t(11;14) (mPFS for TE vs TIE: 23 months vs 22 months, p=0.2137). Multivariate analysis confirmed that 11q gain was an independent adverse factor to PFS of MM with t(11;14) (Hazard ratio: 11.621, 95% CI: 1.027-131.507; p=0.048; n=37).
Conclusions: Gain of 11q13.3-q25 is a reproducible chromosomal copy number abnormality in NDMM characterized by coexisting with t (11;14), complex karyotypes, higher plasma cell ratio in bone marrow and poor treatment response to VRD. t(11;14) patients with 11q gain had a poorer PFS compared to patients with t(11;14) only, mPFS was only 9 months for TIE patients. t(11;14) with 11q gain NDMM patients needs to be considered as high risk and ASCT could improve patients' survival.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal