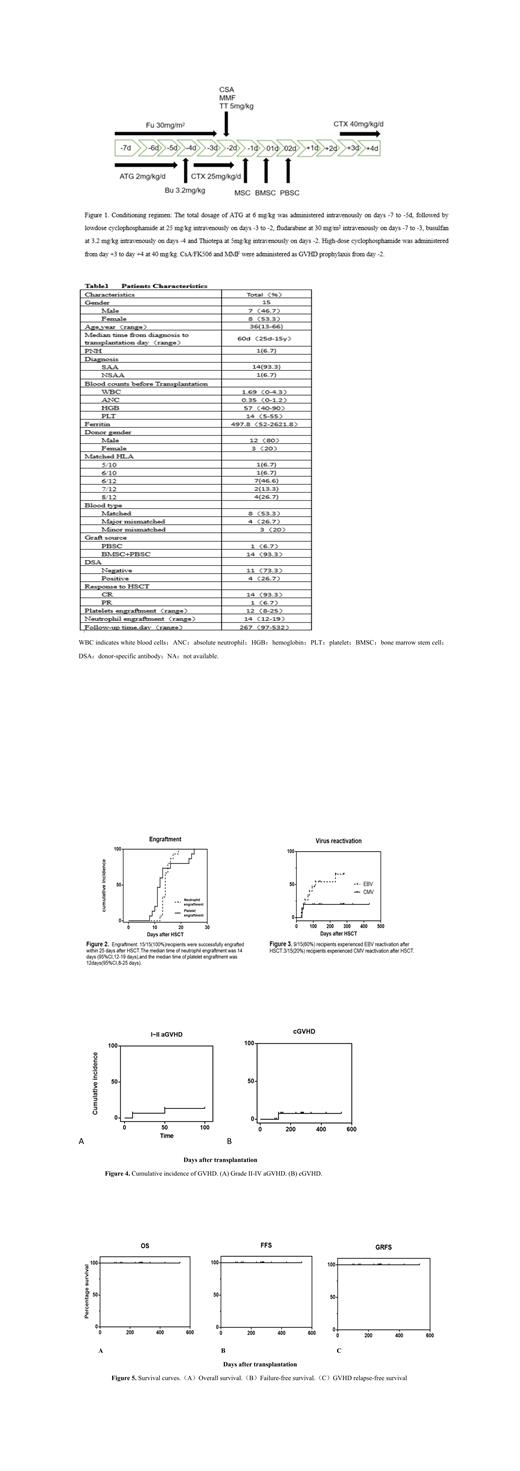
Aplastic anemia (AA) is one of life-threatening hematological disorder that can be cured by hematopoietic stem cell transplantation. When the absence of a matched sibling donor, haploidentical stem cell transplantation (haplo-HSCT) becomes an alternative choice for AA patients. We used a prospective study aimed to confirm the feasibility of Thiotepa-based modified post-transplantation cyclophosphamide (PTCY) strategy in haplo-HSCT for AA patients. We analyzed the outcomes of 15 patients who had undergone haplo-HSCT between October 2021-May 2023 in our clinical center. The modified condition regimen consisted of anti-thymoglobulin/antilymphocyte globulin, fludarabine, thiotepa, busulfan and modified low and high-dose cyclophosphamide, mycophenolate mofetil (MMF) and tacrolimus (FK-506)/cyclosporin (CsA) were administered as graft versus host disease (GVHD) prophylaxis after transplantation. The median follow-up time was 267 (range 97-532) days. All 15 patients were successfully implanted (100%), the median times for neutrophil and platelet engraftment were 14 (range 12-19) days and 12 (range 8-25) days, respectively. But one patient developed secondary graft failure because of infected with COVID-19、cytomegalovirus and H1N1. Fortunately, she subsequently underwent a successful secondary haplo-HSCT. The most common regimen-related toxicity was canker sore, hemorrhagic cystitis occurred in 3 patients during transplantation. The cumulative incidence of grade Ⅰ-Ⅱ aGVHD and cGVHD were 13.3% and 6.7% respectively, whereas the incidence of grade Ⅲ-Ⅳ aGVHD was not found so far. All 15 patients are alive, with a failure-free survival rate of 100% and GVHD relapse-free survival rate of 100%. The efficacy evaluation of haplo-HSCT, 14 patients achieved CR and 1 patient achieved PR. Because of 7 patients received the prevention strategy of Letermovir since August 2022, the reactivation rate of cytomegalovirus (CMV) was only 20% and 60% of Epstein-Barr virus (EBV) reactivation, but the CMV diseases and post-transplantation lymphoproliferative disorder (PTLD) were rare. Our study using Thiotepa-based modified haplo-HSCT demonstrated an encouraging result with prolonged survival and reduced complications for aplastic anemia patients.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal