Introduction: Myelofibrosis (MF) is a type of myeloproliferative neoplasm characterized by bone marrow fibrosis, splenomegaly, progressive anemia, and debilitating constitutional symptoms. Fedratinib (FEDR) is a selective Janus kinase-2 inhibitor (JAK2i) approved for the treatment of adult patients with intermediate (int)-2 or high-risk primary or secondary MF. Patients with MF have a median overall survival of 5-7 years; however, prior to FEDR approval, ruxolitinib (RUX) was the only approved JAK2i for MF treatment, with the majority of patients discontinuing RUX within 3 years of treatment initiation (Harrison C, et al. Ann Hematol 2020;99:1177-1191). Given the current availability and use of FEDR, the primary study objective was to describe demographics, clinical characteristics, and treatment patterns of patients with MF receiving FEDR in real-world practice settings after prior RUX treatment. A secondary objective was to assess changes in MF-related symptoms and spleen size during FEDR treatment.
Methods: We report interim data from a medical records review of adult patients with MF who received FEDR treatment after RUX discontinuation (due to treatment refractoriness, relapse, or intolerance) in Canada (CAN), Germany (GER), and the United Kingdom (UK). Data collection is ongoing, and we present data abstracted from March through May 2023. Patients were required to have an int-2 or high-risk MF diagnosis at FEDR initiation and to have initiated FEDR after date of first availability in each country (CAN: Sep 21, 2020, GER: Feb 9, 2021, UK: Nov 1, 2021) up to 6 months prior to data abstraction. Patients who received allogenic, hematopoietic cell transplantation after initial MF diagnosis or participated in a JAK2i trial were excluded. Spleen size evaluation through palpation at FEDR initiation and at least once within the first 6 months of FEDR use was required. Study outcomes measured were patient characteristics, treatment patterns, MF-related symptoms, and spleen size evaluations. Descriptive statistics are reported.
Results: A total of 58 patients (CAN: 13, GER: 32, UK: 13) were included in the analysis. Median age at MF diagnosis and FEDR initiation was 67.9 and 71.8 years, respectively. 65.5% of patients were male, and 91.4% were White. Most patients were diagnosed with primary MF (60.3%) and had JAK2 V617F mutation (84.5%). Among patients who had a bone marrow biopsy (n = 51), 58.8% had grade 2 bone marrow fibrosis. Mean baseline Charlson Comorbidity Index score was 2.5.
Median time from MF diagnosis and RUX treatment discontinuation to FEDR initiation was 34.0 months and 0.7 months, respectively. Most common reasons for FEDR initiation were splenomegaly (75.9%), RUX failure (67.2%), and to achieve symptom control (63.8%) (Table). Over a median follow-up of 12.1 months after FEDR initiation, 19 patients (32.7%) discontinued FEDR treatment with a median treatment duration of 7.7 months. Among the 39 patients taking FEDR at data abstraction, median treatment duration was 12.5 months.
At FEDR initiation, 48.3% and 51.7% had int-2 risk and high-risk MF, respectively. The most common MF-related symptoms presented were fatigue (74.1%), abdominal discomfort (63.8%), and night sweats (46.6%) (Table). 62.1% of patients initiated FEDR treatment at the recommended therapeutic dose of 400 mg, and 74.1% were receiving 400 mg at end of follow-up/treatment discontinuation. Among patients with ≥ 1 FEDR dose change, titration to therapeutic dose (68.4%) was the most common reason for their first dose change.
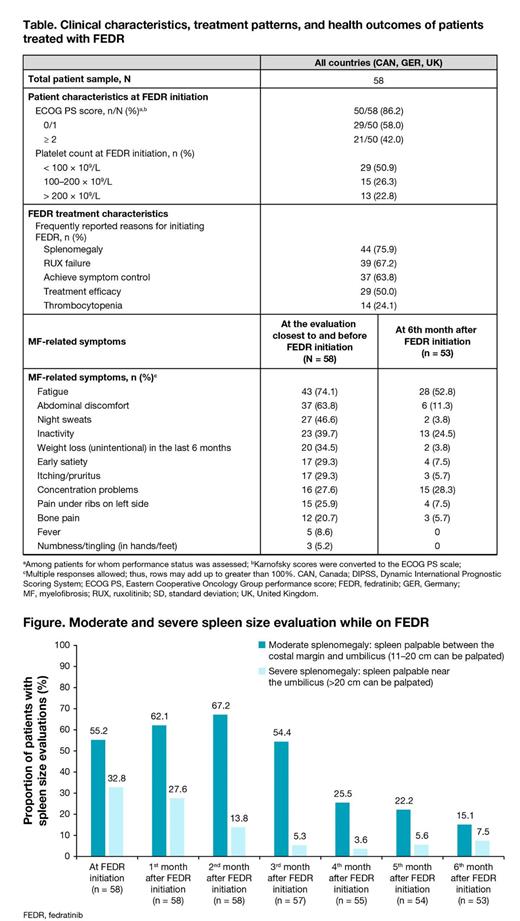
MF-related symptoms decreased in the first 6 months of FEDR treatment, including fatigue (74.1% [at FEDR initiation] reduced to 52.8% [at 6 months after FEDR initiation]), abdominal discomfort (63.8% reduced to 11.3%), and night sweats (46.6% reduced to 3.8%). The proportion of patients with severe (palpable spleen:> 20 cm) and moderate splenomegaly (palpable spleen: 11-20 cm) decreased from FEDR initiation to 6 months after initiation (severe splenomegaly: 32.8% to 7.5%, moderate splenomegaly: 55.2% to 15.1%) (Figure).
Conclusion: Thepatients included in this study exhibited a significant level of illness . In this interim analysis, patients treated with FEDR following RUX treatment failure showed resolution of MF-related symptoms and a marked decrease in splenomegaly in the initial 6-month period, demonstrating the real-world effectiveness of FEDR treatment in patients with MF.
Disclosures
Passamonti:Novartis: Honoraria; GSK: Honoraria; Bristol Myers Squibb / Celgene: Honoraria; Sierra Oncology: Honoraria; AbbVie: Honoraria; Janssen: Honoraria; Roche: Honoraria; AOP Orphan: Honoraria; Karyiopharma: Honoraria; Kyowa Kirin and MEI: Honoraria; Sumitomo: Honoraria. Parikh:RTI Health Solutions: Current Employment; Bristol Myers Squibb: Research Funding. Korgaonkar:RTI Health Solutions: Current Employment; Bristol Myers Squibb: Research Funding. Chevli:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Slaff:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Rombi:RTI Health Solutions: Current Employment; Qualworld: Ended employment in the past 24 months. Adeyemi:Bristol Myers Squibb: Current Employment; pfizer: Current equity holder in publicly-traded company. Davis:RTI Health Solutions: Current Employment; Astrazeneca: Research Funding; BMS: Research Funding; Novartis: Research Funding; Pfizer: Research Funding; Eisai: Research Funding; Merck: Research Funding; Sobi: Research Funding; Gilead: Research Funding. Yucel:Bristol Myers Squibb: Current Employment, Current holder of stock options in a privately-held company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal