Background: Allo-HCT is the only potentially curative therapy available for many hematologic malignancies, but due to its intensity and associated toxicities, is infrequently offered to older adults. This study explored the feasibility and efficacy of individualized, geriatric assessment (GA)-guided intervention among older adult candidates for allo-HCT.
Methods: Patients ≥ 60 years old that were candidates for allo-HCT were recruited between June 11, 2021 and July 1, 2022. Exclusion criteria included inability to speak English, diagnosis of dementia, and planned allo-HCT in < 1 month or > 6 months. Baseline GA was completed at time of enrollment. Subjects were prescribed multi-domain personalized geriatric intervention based on results of their individual GA. Patients were continued on geriatric intervention for up to 6 months or until transplant. A post-intervention GA was conducted after 6 months or at time of admission for allo-HCT, whichever occurred first. Metrics included in GA are defined in table 1. Subjects were provided wearable accelerometer device for activity tracking. Primary outcome of interest was change in 6-minute walk test (6MWT) and secondary outcomes included improved or maintained short physical performance battery (SPPB), receipt of allo-HCT, and overall survival (OS). Wilcoxon signed rank test was used to compare GA metrics pre- and post-intervention, and OS was estimated using the Kaplan-Meier method. Exploratory analyses included univariable modeling of association of baseline GA metrics with receipt of allo-HCT and OS.
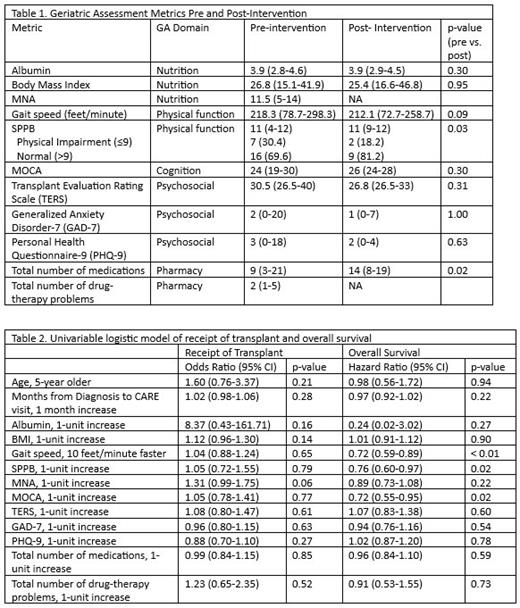
Results: A total of 63 allo-HCT eligible patients were approached, 30 consented and were evaluable. Of 33 not enrolled, 17 declined (7 due to requirement to wear accelerometer device), 7 were planned for allo-HCT in < 1 month, 4 were not interested in allo-HCT, 3 had virtual appointments with incomplete GA, 1 had dementia and 1 with anticipated allo-HCT date > 6 months. The majority of enrolled subjects were male (80%) and all were non-Hispanic, white. Median age at enrollment was 68.9 years (range: 60.8-79.2). Half (n=15) were diagnosed with acute myeloid leukemia, 40% (n=12) with myelodysplastic syndrome, and one each with chronic myelomonocytic leukemia, myelofibrosis, and non-Hodgkin lymphoma. The median time from allo-HCT-eligible diagnosis to baseline GA was 4.1 months (range: 1.2-118.9). More than half (n=16, 53.3%) of subjects received allo-HCT. Median time to allo-HCT from diagnosis was 13.8 months (range: 3.3-122.1) and from baseline GA was 4.6 months (range: 0.5-10.8). No one baseline GA metric was associated with receipt of allo-HCT (table 2). Median OS from study enrollment for all subjects was not reached (95% CI: 9.8 month-NR) with median follow-up of 14.8 months (range: 10.7-19.3). At baseline, better gait speed on 6MWT (HR: 0.72; 95% CI: 0.59-0.89, p=0.0018), SPPB (HR: 0.76; 95% CI: 0.60-0.97; p=0.02), and Montreal Cognitive Assessment (MOCA; HR: 0.72; 95% CI: 0.55-0.95; p = 0.02) scores were all associated with improved OS in univariable analysis (table 2).
Pre and post-intervention GA metrics are presented in table 1. Only 14 subjects completed at least partial post-intervention GA, 12 of whom received allo-HCT. Reasons for not completing post-intervention GA included subjects declining to schedule (n=6), issues of care coordination (e.g.: frequent or prolonged hospital admissions, changes to allo-HCT admission plan; n=6), and death (n=4). Twelve subjects had both pre and post-intervention 6MWT data, 83.3% (n=10) improved by at least 50 feet. Ten subjects had both pre and post-intervention SPPB and all improved by at least 1 point or maintained a normal score (>9).
Discussion: Improvement in or maintenance of 6MWT and SPPB were demonstrated in the majority of subjects with measured pre and post-intervention GA. Unfortunately, attrition was a major limitation of the study. Patterns of loss to follow-up, as well as reasons for screen failure, will be important areas for further exploration and potential targets for intervention at institutional and provider levels. Importantly, age was not associated with OS. Early education about allo-HCT paired with patient-centered goals of care conversations may be useful in identifying older adults most motivated and likely to benefit from GA-guided intervention.
Disclosures
Wall:Abbvie, BMS: Honoraria; CTI: Speakers Bureau. Presley:Jazz Pharmaceuticals: Consultancy; Targeted Oncology: Consultancy. Artz:Abbvie: Consultancy; Magenta Therapeutics: Other: Advisory Board; Astra Zeneca: Other: Advisory Board; Radiology Partner: Current equity holder in private company, Other: Spouse equity interest. Rosko:Sanofi: Research Funding; FDA ODAC Committee: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal