Background: Essential thrombocythemia (ET) is a myeloproliferative neoplasm (MPN) characterized by clonal blood cell proliferation, excessive platelet production, constitutional symptoms, and increased risk of vascular complications, as well as a risk of progression to myelofibrosis (MF). Previous prognostic studies of patients with ET have largely been based on retrospective analyses, and limited prospective data exist regarding disease progression. The prospective Myelofibrosis and Essential Thrombocythemia Observational Study (MOST) collected real-world data from patients with ET. This analysis evaluates disease progression in patients with ET enrolled in MOST.
Methods: MOST (NCT02953704) enrolled patients with ET aged ≥60 years, or with a history of thromboembolic events, or who were receiving ET-directed therapy (excluding aspirin only). Baseline clinical characteristics data were collected as previously described (Yacoub A. CLML. 2021). For this analysis, progression from ET to MF was defined as meeting 1 or more of the following criteria during the study period: 1) Bone marrow biopsy with fibrosis grade ≥2 or pathologic diagnosis of MF; 2) death owing to MF/myelodysplastic syndrome/acute myeloid leukemia; 3) circulating blasts >1% and new/worsening splenomegaly (SPM); 4)new/worsening SPM and ≥2 of white blood cell (WBC) count >11×10 9/L, hemoglobin (Hb) <10 g/dL, and platelet count <100×10 9/L. Median (range) follow-up was 57.3 months (42-70).
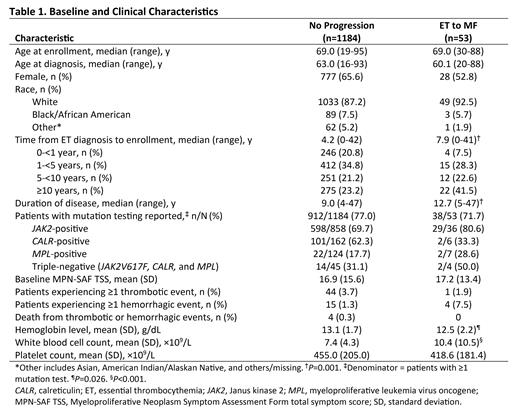
Results: Of the 1237 patients with ET enrolled in MOST; 53 (4.3%) met criteria for progression to MF. Among patients with progression, most had progressed due to fibrosis (criteria 1, 56.6%); 15.1%, 11.3%, and 28.3% of patients progressed due to criteria 2, 3, and 4, respectively. Notably, only 176 patients (14.2%) enrolled in the study had bone marrow data available. Comparison of the enrollment characteristics of patients with and without progression identified no differences in age at enrollment or diagnosis (Table 1). However, time from diagnosis to enrollment and from diagnosis to end of study were significantly longer in patients with progression than those without (median 7.9 vs 4.2 years, median 12.7 vs 9.0 years, respectively; both P=0.001). Of all enrolled patients with vs without progression tested for known driver mutations, a higher percentage with progression were JAK2-positive vs those without progression (80.6% [29/36] vs 69.7% [598/858]). At enrollment, the percentages of patients with vs without progression receiving ET-directed therapies were similar (94.3% vs 94.8%). Among patients with vs without progression, mean baseline Hb was significantly lower (12.5 g/dL vs 13.1 g/dL; P=0.026), mean WBC counts were significantly higher (10.4×10 9/L vs 7.4×10 9/L; P<0.001), and mean platelet counts were comparable (418.6×10 9/L vs 455.0×10 9/L; P=0.466). MPN-SAF TSS was similar for patients with vs without progression at baseline. Fewer patients with vs without progression had ≥1 thrombotic events (1.9% [1/53] vs 3.7% [44/1184]) during the study, whereas a higher percentage of patients had ≥1 hemorrhagic events (7.5% [4/53] vs 1.3% [15/1184]).
Conclusions: This real-world analysis of data from MOST showed that 4.3% of patients with ET progressed to MF during the study period. Compared with patients without progression, patients with ET to MF progression had longer duration of disease, higher WBC count, and lower Hb at enrollment; symptom burden at enrollment was similar between the 2 groups. One limitation of this study is the lack of bone marrow data for the majority of patients, suggesting a possible underestimation of progression to MF. These findings and further analysis of MOST data will add insight into disease progression in patients with ET and facilitate clinical management of this patient population.
Disclosures
Yacoub:Acceleron Pharma, Inc.: Consultancy; Apellis: Consultancy; Gilead: Consultancy; Novartis: Consultancy; Pfizer: Consultancy; Pharmaessentia: Consultancy; CTI Pharma: Consultancy; Protagonist Therapeutics, Inc.: Consultancy; Incyte Corporation: Consultancy; Servier: Consultancy; Notable Labs: Consultancy; AbbVie Inc.: Consultancy; AbbVie, Acceleron, Apellis, CTI Pharma, Gilead, Incyte, Notable Labs, Novartis, Pfizer, PharmaEssentia, Servier.: Consultancy. Lyons:Texas Oncology: Current holder of stock options in a privately-held company; Astellas Pharma: Research Funding; Lessen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte Corporation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; McKesson: Other: Leadership; Exact Sciences: Research Funding; Pfizer: Research Funding; Bayer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Braunstein:Incyte Corporation: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Hamer-Maansson:Incyte Corporation: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Kalafut:Incyte Corporation: Current Employment, Current equity holder in publicly-traded company. Mesa:Sierra Onc: Consultancy; Incyte: Research Funding; Abbvie: Research Funding; Mays Cancer Center: Research Funding; Genetech: Research Funding; Constellation: Consultancy, Research Funding; Novartis: Consultancy; Promedior: Research Funding; Celgene: Research Funding; CTI BioPharma., a Sobi Company: Research Funding; Samus: Research Funding; LaJolla Pharma: Consultancy; NCI: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal