Background: Hereditary spherocytosis (HS) is caused by pathogenic variants in genes encoding red blood cell (RBC) cytoskeletal and (trans)membrane proteins. This ultimately leads to instability of the RBC membrane and subsequent membrane loss, resulting in the transformation of the biconcave RBC into a poorly deformable spherocyte, which is susceptible to premature splenic clearance. The diagnosis of HS has substantially improved over the last years and new advanced techniques have been established, including osmotic gradient ektacytometry, eosin-5'-maleimide (EMA) binding test and next-generation sequencing (NGS). However, there is little knowledge on the applicability of these diagnostic outcome parameters as biomarkers for clinical severity in HS.
Aim: To explore possible correlations between established and novel diagnostic outcome parameters and clinical severity in a well-defined cohort of HS patients.
Methods: This monocenter retrospective cohort study involves patients diagnosed with HS upon referral to our department between 2012 and 2023. Clinical characteristics, routine and advanced diagnostic laboratory parameters, and genotype were evaluated. Clinical severity was classified as previously described, based on parameters such as hemoglobin, reticulocyte percentage and bilirubin 1. The following laboratory tests were included: routine hematological parameters (Cel-Dyn Sapphire or Alinity hq), EMA binding test, osmotic fragility test (OFT) and osmotic gradient ektacytometry (osmoscan, Lorrca MaxSis). The latter technique was also explored for other parameters than the commonly used EI max, O hyper and O min. NGS gene panel analysis was used for molecular diagnosis. Differences between groups were determined using either Chi-squared test, unpaired t-test or Mann-Whitney U test and correlations using point-biserial, Pearson's or Spearman's test via GraphPad Prism.
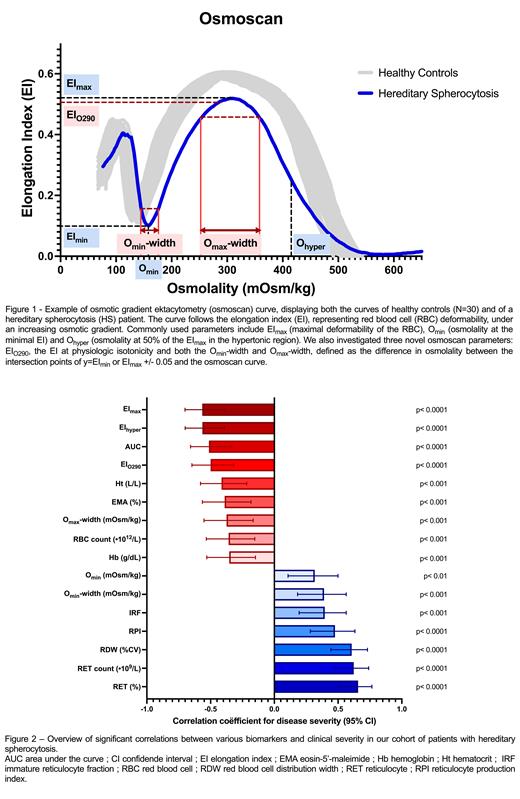
Results: Eighty-two non-splenectomized patients were included. Thirty-five of these (42.7%) were classified as mild, and forty-seven as moderate-severe (57.3%)). Demographic characteristics nor genotype differed significantly, albeit SPTB variants occurred more frequently in the moderate-severe group (15 vs. 4 in the mild group). Expectedly, both reticulocyte count and EI max correlated with clinical severity. RBC distribution width (RDW) correlated moderately, suggesting greater RBC heterogeneity (r=0.606, p<0.0001) is associated with a more severe clinical phenotype. Similarly, EMA binding test results correlated with clinical severity (r=0.390, p<0.001), indicating more pronounced membrane loss in clinically more severe patients. We further evaluated three novel osmoscan parameters ( Figure 1): EI O290, the elongation index (EI) at physiologic isotonicity, which correlated with clinical severity (r=-0.500, p<0.0001), and also with EI max (r=0.856, p<0.0001). The O min-width and O max-width, defined as the difference in osmolality between the intersection points of y=EI min or EI max +/- 0.05 and the osmoscan curve. Thisassesses the effect of changes in osmolality on EI min or EI max, respectively. O min-width correlated with RDW (r-0.439, p<0.0001), OFT results (N=30, r=0.671, p<0.0001) and clinical severity (r=0.390, p<0.001). O max-width correlated with clinical severity (r=-0.375, p<0.001) and strongly with percentage of hyperchromic cells (N=76, r=-0.751, p<0.0001), yet weakly with RDW (-0.273, p<0.05). All significant correlations with clinical severity are displayed in Figure 2.
Conclusion: In this study, a number of routine and advanceddiagnostic laboratory parameters were found to correlate with clinical severity in HS. O min-width and O max-width are novel osmoscan-derived biomarkers that correlated with RDW, hyperchromic cells (O max-width), OFT results (O min-width) and clinical severity. EI O290, another novel osmoscan-derived biomarker, also correlated with clinical severity, although not stronger than EI max and AUC. The biomarkers identified in this study, together with patient-reported outcome measures (PROMs), could be used to revise the previously established severity classification system and thereby aid in stratifying clinical severity and subsequent clinical decision-making.
References:
1. Eber, S. W., Armbrust, R., & Schröter, W. (1990).
Disclosures
De Wilde:Agios Pharmaceuticals, Inc.: Research Funding. Van Beers:RR Mechatronics: Research Funding; Novartis AG: Research Funding; Pfizer, Inc.: Research Funding; Agios Pharmaceuticals, Inc. Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Rab:Axcella Therapeutics, Inc.: Research Funding; RR Mechatronics: Research Funding; Pfizer, Inc.: Research Funding; Agios Pharmaceuticals, Inc.: Consultancy, Research Funding. van Wijk:Pfizer, Inc.: Consultancy, Research Funding; Agios Pharmaceuticals, Inc.: Consultancy, Research Funding; Axcella Therapeutics, Inc.: Research Funding; RR Mechatronics: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal