Introduction: Moderate iron deficiency anemia(IDA), defined as a hemoglobin below 11 g/dl, occurs in 1.5% of the United States population and is overrepresented in minority populations. IDA is known to cause permanent structural and function damage in children, but IDA's cognitive and neurovascular phenotype in adults is poorly characterized. We performed brain MRI and cognitive testing in 34 otherwise healthy women with IDA to determine whether oxygen delivery and metabolism are preserved as well as the association of IDA with brain volumes and cognitive function.
Methods: We recruited potential blood donors from four hospital-based donor centers, Children's Hospital Los Angeles, University of California Los Angeles, Cedar's Sinai, and City of Hope, whose point-of-care hemoglobin values were less than 10.5 g/dl. We also recruited individuals from the community using social media advertisements, with anemia documented by screening hemoglobin assessment. All participants were free from inflammatory, infectious, or malignant diseases that may impact blood counts and iron metabolism. IDA was confirmed by laboratory assessment of CBC, reticulocyte count, iron indices, methyl malonic acid, hemoglobin electrophoresis, homocysteine, and high-sensitivity C-reactive protein. Patients underwent a four-hour neurocognitive assessment consisting of subsets from the Weschler Abbreviated Scale of Intelligence (WASI-II), the California Verbal Learning Test (CVLT), the Rey Complex Figure Test (RCFT), and the NIH Cognitive Toolkit. MRI was performed on a 3T Philips Achieva using a 32-element head coil. Anatomic imaging consisted of 3D T1, 3D T2, 3D T2*/QSM, and 2D multishell diffusion imaging. Phase contrast and arterial spin labeling measured total and regional brain blood flow, respectively. Cerebral venous oximetry was performed using T2 relaxation under spin tagging.
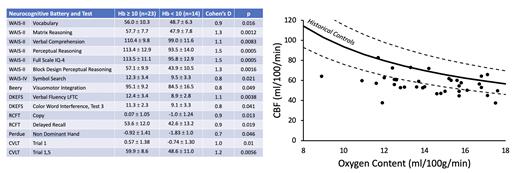
Results: The study population demonstrated a classic IDA phenotype characterized by low ferritin and transferrin saturation, high iron binding capacity, decreased MCV and MCHC, and hypochromic microcytosis on blood smear (not shown). Neurocognitive function was impaired across multiple domains for women having hemoglobin values less than 10.0 g/dl (Figure 1, left), with Cohen's D values ranging from 0.7 - 1.5. Cerebral blood flow (CBF) rose slightly as oxygen content declined (Figure 1, right), but much less than predicted based on historical controls 1,2 leading to impaired brain oxygen delivery. Oxygen extraction fraction was independent of hemoglobin concentration, thus cerebral metabolic rate was also decreased. Grey matter volume was smaller in the right temporal lobe and correlated with 2/3 of the abnormal cognitive indices (in Figure 1, left). White matter volume was decreased in the right cingulate gyrus, corpus callosum, and cerebellum, correlating with the remaining abnormal cognitive indices.
Discussion: Our study demonstrates that iron deficiency has serious effects on cognitive performance; individuals with hemoglobin less than 10.0 g/dl scored more than one standard deviation below their peers with milder anemia. Poor cognitive performance was correlated with demonstrable brain shrinkage whose reversibility is unknown. The failure of the brain to upregulate CBF in response to IDA was striking because most patients with chronic anemia preserve cerebral oxygen delivery through compensatory hyperemia 1,2. While both anemia and iron deficiency can impair brain function on their own, it is likely that iron deficiency is the primary contributor to the cognitive and neurovascular effects. We will test this hypothesis in subsequent work by reexamining women with IDA shortly after intravenous iron administration, thus creating a window where the iron deficiency has been corrected but the anemia persists(NCT05929729). We will also determine whether the cerebrovascular, anatomic, and functional deficits are reversible with iron repletion.
References
1. Brown MM, Marshall J. Regulation of cerebral blood flow in response to changes in blood viscosity. Lancet. 1985;1(8429):604-609.
2. Bush AM, Borzage MT, Choi S, et al. Determinants of resting cerebral blood flow in sickle cell disease. Am J Hematol. 2016;91(9):912-917.
Disclosures
Wood:Philips Medical Systems: Other: Support-In-Kind, Research Funding; Imago Biosciences: Consultancy; Hillhurst: Consultancy; Agios: Consultancy; Celgene: Consultancy; Pharmacosmos: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal