Background:
Platelets play a critical role in physiological processes by sensing the extracellular environment through their membrane proteins. Changing the quantity or quality of these proteins can profoundly affect platelet physiology and impact health. The native membrane environment provides essential additional regulatory cues that impact the protein structure and mechanism of action.
Single-particle cryogenic electron microscopy (cryo-EM) has transformed structural biology by allowing high-resolution structures of membrane proteins and large macromolecules to be solved from highly purified, homogeneous samples. Our recent breakthroughs in data processing make it feasible to obtain high-resolution protein structures from crude preparations in their native environments by integrating cryo-EM with the novel “Build and Retrieve” (BaR) data processing methodology. By performing in silico purification, image sorting, and model building from large heterogenous datasets, this iterative bottom-up methodology opens new avenues for an in-depth systems biology approach with structural biology that will uncover how the native environment, including lipid and metal binding, post-translational modifications, and co-factor associations regulate platelet function at the molecular level.
Method:
Whole blood was collected, and the platelet membrane proteins were solubilized by 1% n-Dodecyl-β-D-Maltoside (DDM) and incorporated into lipidic nanodisc MSP1E3D1 directly from the endogenous source. After being enriched by size exclusion chromatography, the mixture of proteins at ~200 kDa was directly applied on cryo-EM grids. Data were collected on Titan Krios G3i cryogenic transmission electron microscope equipped with a BioQuantum K3 camera. All data processing was done in cryoSPARC using the BaR protocol. The near-atomic-resolution cryo-EM maps were used for protein identification using the program phenix.sequence_from_map in the PHENIX suite. The final protein models were built by Coot and refined by PHENIX.
Results:
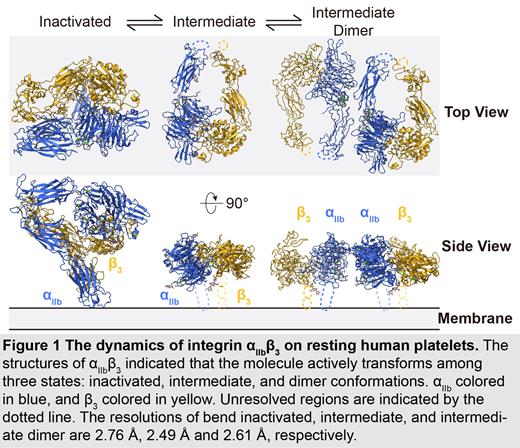
We have used using cryo-EM followed by the BaR method to solve the first unmodified integrin α IIbβ 3 structure directly from resting human platelet membranes in its inactivated and intermediate states at 2.76Å and 2.49Å, respectively. From these two structures, we observed the ion binding sites at the β1-domain and β-propeller domain in agreement with previous “classical” α IIbβ 3 structural studies using a purified sample. More importantly, we were able to assign endogenous N-linked glycan sites, which reflected how glycosylation naturally regulates α IIbβ 3 dynamic and function on resting human platelets. Finally, we also solved a novel third unique dimer conformation of α IIbβ 3 at 2.61Å formed by two intermediate-states of α IIbβ 3. This may indicate a previously unknown self-regulatory mechanism of α IIbβ 3 in its native environment.
Conclusion:
In conclusion, our data show the power of using cryo-EM with the BaR method to uncover novel structures directly from natural sources to identify unrecognized regulation mechanisms for proteins without artifacts due to purification processes. A distinct advantage of the BaR method is that it is an unbiased approach for solving multiple structures from raw samples, which highlights the potential of building a protein structural atlas from platelets. These data have the potential to enrich our understanding of platelet signaling circuitry and foster the development of higher-quality diagnoses and treatments for patients with platelet disorders.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal