Background:
Venetoclax in combination with hypomethylating agents or low dose cytarabine is a safe and effective treatment for newly diagnosed older, unfit patients with acute myeloid anemia (AML). Combining venetoclax with high intensity combination chemotherapy may increase response rates and eventual chance for cure. Previous studies have combined venetoclax with fludarabine, cytarabine, idarubicin, and G-CSF (FLAG-ida) with 89% of patients achieving a composite complete remission (CR), of which 93% were MRD negative (DiNardo et. al, AJH, 2022). We report the preliminary results of a phase 1 study examining the addition of venetoclax to a high intensity induction regimen, CLAG-M (cladribine, cytarabine, mitoxantrone, G-CSF), for fit patients with newly diagnosed adverse risk or relapsed/refractory high-grade myeloid neoplasms.
Methods:
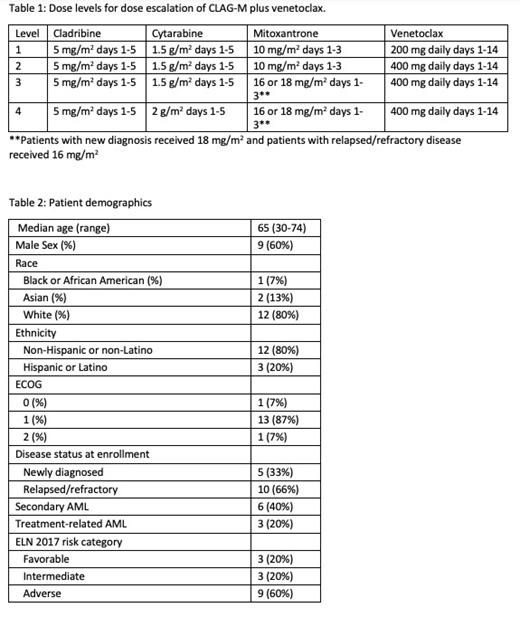
Subjects with AML or other high-grade myeloid neoplasm (≥10% myeloid blasts in peripheral blood or marrow) were eligible if they had (1) new diagnosis with adverse risk disease by European LeukemiaNet 2017 criteria or (2) relapsed/refractory (R/R) disease. Venetoclax was administered on day 1-14 with a 4-day ramp-up. All patients received standard neutropenic prophylaxis and venetoclax was dose-adjusted for concomitant use of CYP3A4 inhibitors. Dose level (DL) is defined in Table 1. Dose limiting toxicity (DLT) was defined as grade >4 non-heme toxicity, prolonged aplasia > 49 days, or grade >3 non-heme toxicity lasting >48h and resulting in a treatment delay beyond day 56. Two subjects were started at DL 2 and a Bayesian optimal interval (BOIN) design was used for subsequent doses, with a true targeted DLT rate of 30%. Re-induction was allowed for persistent disease after the first cycle; subjects with CR were eligible to receive up to three cycles of venetoclax plus cladribine and cytarabine (CLAG) at the same dose level as induction. Target accrual is 20 subjects. Events were defined as death, relapse, or failure to achieve CR/CRi on post induction evaluation. The study was approved by the local IRB, and subjects were consented in accordance with the Declaration of Helsinki.
Results:
At time of data cutoff (7/6/23), 15 subjects (5 with newly diagnosed and 10 with R/R AML) were enrolled. Median age was 65 (range: 30-74) years. Five were newly diagnosed and 10 were R/R. Two subjects were enrolled at DL 1, 6 at DL 2, 5 at DL 3, and 2 at DL 4 as per the BOIN design. Two DLTs occurred: grade 4 respiratory failure at DL 2 and grade 5 sepsis at DL 3. Treatment related grade 3 adverse events (AE) included febrile neutropenia (5 subjects), dyspnea (2), mucositis (1), hypoxia (1), delirium (1), syncope (1), tumor lysis syndrome (1), and sepsis (1). Non-treatment related grade 3 AEs included hypokalemia (1 patient) and pericarditis (1). 28-day mortality was 7%. Twelve subjects received 1 cycle and 3 subjects received 2. Overall response rate (CR + CRi) was 73%. Two subjects achieved CR without MRD, 1 CR with MRD, 3 CRi without MRD, 5 CRi with MRD, 1 MLFS without MRD, 2 had persistent disease, and 1 died before evaluation. Median overall survival from treatment start was 7.4 months (95% CI 4.2 months - not reached [NR]). Median follow up of survivors was 6 months. Median event free survival was 3.3 months (95% CI 1.2 months - NR). Four subjects (1 newly diagnosed, 3 R/R) went on to receive allogeneic hematopoietic cell transplant (HCT). The administration of venetoclax presented challenges for inpatient and outpatient dosing, as medication diaries showed subjects underdosing venetoclax when taking concomitant posaconazole due to the complexity of the dosing regimen.
Discussion:
Preliminary results show that venetoclax can be safely added to a high intensity regimen for fit, high-risk patients without adversely affecting standard therapies including subsequent allogeneic HCT. Grade 3 and higher toxicity was common. In this preliminary analysis, the overall response rate was 73%, which is comparable to that observed with other chemotherapy regimens with the addition of venetoclax. A protocol amendment is in process to simplify venetoclax dosing with concomitant CYP3A4 inhibitors. This study will ultimately determine the MTD for subsequent phase II study to evaluate the therapeutic efficacy of venetoclax and CLAG-M.
OffLabel Disclosure:
Raychaudhuri:Moderna: Current equity holder in publicly-traded company; Pfizer: Current equity holder in publicly-traded company; Biontech: Current equity holder in publicly-traded company. Halpern:Abbie, Notable Labs, Agios: Consultancy; Imago Bioscience, Bayer, Gilead, Jazz, Incyte, Karyopharm Therapeutics, Disc Medicine: Research Funding. Appelbaum:2seventy bio: Research Funding. Cassaday:Incyte: Research Funding; Merck: Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Kite/Gilead: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Servier: Research Funding; Vanda Pharmaceuticals: Research Funding; Jazz: Consultancy, Honoraria; Autolus: Membership on an entity's Board of Directors or advisory committees; PeproMene Bio: Membership on an entity's Board of Directors or advisory committees; Seagen: Other: Spouse was employed by and owned stock in Seagen within the last 24 months.. Walter:Abbvie, Adicet, Amphivena, BerGenBio, Bristol Myers Squibb, GlaxoSmithKline, Orum: Consultancy; Amgen, Aptevo, Celgene, Janssen, Jazz, MacroGenics, Pfizer: Research Funding; ImmunoGen, Jura: Consultancy, Research Funding. Percival:Abbvie: Research Funding; Ascentage: Research Funding; Astex: Research Funding; Biosight: Research Funding; BMS: Research Funding; Glycomimetics: Research Funding; Pfizer: Research Funding; Telios: Research Funding.
Venetoclax is a BCL2-inhibitor that is FDA approved in combination with azacitidine, decitabine, or low-dose cytarabine for newly-diagnosed acute myeloid leukemia in adults 75 years or older, or who are unfit for intensive chemotherapy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal