Background: Mosunetuzumab is a CD20xCD3 bispecific antibody that is approved for the intravenous (IV) treatment of patients with relapsed/refractory (R/R) follicular lymphoma (FL) who have received at least two prior lines of systemic therapy, and is suitable for administration in the outpatient setting. In an ongoing Phase I/II study (NCT02500407), IV mosunetuzumab induced durable responses and had a manageable safety profile in patients with heavily pre-treated R/R indolent and aggressive B-cell non-Hodgkin lymphomas (NHL) when administered as a fixed-duration treatment (Budde et al. J Clin Oncol 2022). Subcutaneous (SC) dosing was also evaluated and showed similar efficacy to IV dosing and a manageable safety profile (Budde et al. ASH 2022). MorningSun (NCT05207670) is an ongoing, open-label, multicenter, Phase II study evaluating the efficacy, safety and pharmacokinetics of SC mosunetuzumab in patients with selected B-cell NHLs, including those without prior treatment (Flinn et al. ASCO 2022). For the first time, we present initial efficacy and safety data for patients with previously untreated, low-tumor burden FL who were enrolled into MorningSun at community and academic centers in the United States.
Methods: Inclusion criteria include previously untreated Grade (Gr) 1 or 2 FL, low tumor burden by GELF criteria, Ann Arbor stage III or IV disease, and ECOG performance status 0-2. Mosunetuzumab is administered without mandatory hospitalization by SC injection in 21-day cycles. In Cycle (C) 1, mosunetuzumab is given on Day (D) 1 (5mg), D8 (45mg) and D15 (45mg). In C2+, mosunetuzumab is given on D1 only (45mg). Treatment is continued for up to 17 cycles (~1 year). Patients who are in complete metabolic response (CMR) at C8 can discontinue active treatment and enter long-term follow-up. Premedication with dexamethasone (20mg) is mandatory in C1 and C2 and optional thereafter. Acetaminophen and diphenhydramine may also be given. The primary endpoint is the rate of progression-free survival at 24 months.
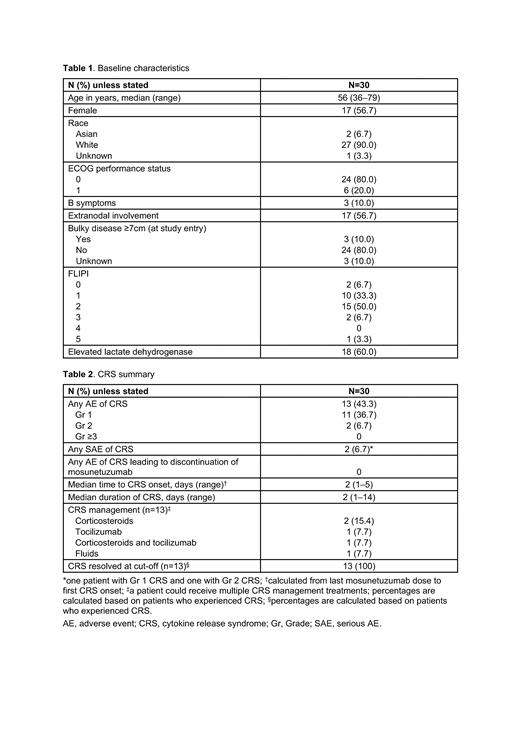
Results: At data cut-off (March 6, 2023), 30 patients had been enrolled (19 at community centers and 11 at academic centers). Median age was 56 years. All patients had ECOG performance status 0-1, and a minority had B symptoms ( Table 1).
The median duration of follow-up was 4.2 months (range: 0.2-11.1) and the median number of cycles received was 7 (range: 1-13). Among 24 patients who had at least one post-baseline tumor assessment, 23 achieved an objective response (95.8%; 95% CI: 78.9-99.9), including 20 who achieved a CMR (83.3%; 95% CI: 62.6-95.3). All responses were observed at the first post-baseline tumor assessment in C4 (median time to response: 2.6 months; 95% CI: 2.6-2.8). At cut-off, responses were ongoing in all patients who achieved CMR and 2 of 3 patients who achieved partial metabolic response.
The most common adverse event (AE) was injection site reaction (53.3% of patients), all of which were Gr 1 (43.3%) or Gr 2 (10.0%). Cytokine release syndrome (CRS) occurred in 43.3%. Most patients had Gr 1 CRS ( Table 2). No Gr ≥3 CRS events or discontinuations due to CRS were reported. CRS occurred in C1 only. All CRS events resolved. Other common (>20%) AEs were headache (50.0%), nausea (30.0%), fatigue (26.7%), pyrexia (23.3%), chills (23.3%) and alanine aminotransferase increased (23.3%). Gr ≥3 AEs in ≥5% of patients were neutrophil count decreased (2 patients; 6.7%). Serious AEs (SAEs) in ≥5% were CRS (2 patients; 6.7%). No SAEs of infection were reported. AEs potentially consistent with immune effector cell-associated neurotoxicity syndrome (ICANS) occurred in 2 patients (Gr 1 confusional state and Gr 1 memory impairment in one patient each); both events resolved. No mosunetuzumab-related AEs leading to discontinuation occurred and no Gr 5 (fatal) AEs were reported.
Conclusions: Initial MorningSun data support that SC mosunetuzumab is active in patients with previously untreated, low-tumor burden FL. Safety data demonstrate a manageable safety profile that is consistent with that seen in patients with R/R B-cell NHLs and is supportive of outpatient administration.
OffLabel Disclosure:
Flinn:Secura Bio: Consultancy; Novartis: Consultancy; Myeloid Therapeutics: Consultancy; Servier Pharma: Consultancy; Kite: Consultancy; Innocare Pharma: Consultancy; BeiGene: Consultancy; AbbVie: Consultancy; Genmab: Consultancy; Hutchinson MediPharma: Consultancy; Genentech: Consultancy; Century Therapeutics: Consultancy; TG Therapeutics: Consultancy; Vincerx Pharma: Consultancy. Budde:Amgen: Research Funding; AstraZeneca: Consultancy, Research Funding; Roche: Consultancy; Merck: Research Funding; ADC Therapeutics: Consultancy; MustangBio: Research Funding; Novartis, Gilead, F. Hoffmann-La Roche Ltd, BeiGene, Genentech, Inc.: Consultancy. Burke:Bayer HealthCare Pharmaceuticals: Consultancy; Verastem: Consultancy; Kymera: Consultancy; MorphoSys AG: Consultancy; Kura Oncology: Consultancy; BeiGene: Consultancy, Speakers Bureau; Epizyme: Consultancy; X4 Pharmaceuticals: Consultancy; Roche/Genentech: Consultancy; Bristol Myers Squibb: Consultancy; AstraZeneca: Consultancy; Adaptive Biotechnologies: Consultancy; Seagen Inc.: Consultancy, Speakers Bureau; Nurix: Consultancy; Morphosys: Research Funding; Gilead Sciences: Consultancy; AbbVie: Consultancy. Anz:Tennessee Oncology: Current Employment; Research is conducted through Sarah Cannon Research Institute with all payments going to SCRI and not individual physicians: Research Funding. Peles:Florida Cancer Specialists: Current Employment; American Oncology Network: Current equity holder in publicly-traded company; Florida Cancer Specialists: Current equity holder in private company; Sarah Cannon Research Institute: Research Funding; Karyopharm: Honoraria; Karyopharm: Speakers Bureau; Florida Cancer Specialists: Membership on an entity's Board of Directors or advisory committees. Sharman:Merck, Novartis: Consultancy; AbbVie, AstraZeneca, BMS, Beigene, Lilly, Genentech, Inc., Genmab: Consultancy; AbbVie, AstraZeneca, BeiGene, BMS, Genentech, Inc., Lilly: Consultancy; Seattle Genetics: Research Funding. Tumula:Texas Oncology: Current Employment. Biondo:F. Hoffmann-La Roche Ltd-: Current holder of stock options in a privately-held company; Genentech, Inc.: Current Employment; Genentech, Inc.: Ended employment in the past 24 months. Jani:Genentech, Inc.: Current Employment; Genentech, Inc. stock (RSUs): Current holder of stock options in a privately-held company; Genentech, Inc. (stock RSUs): Current equity holder in publicly-traded company. Wu:Genentech, Inc.: Current Employment; F. Hoffmann-La Roche Ltd: Current equity holder in publicly-traded company. Lin:Genentech, Inc., GSK, AstraZeneca, Pfizer: Current equity holder in publicly-traded company; Genentech, Inc.: Current Employment. Parmar:Genentech, Inc.: Current Employment; Genentech, Inc.: Current holder of stock options in a privately-held company. Mun:FTE at Genentech, Inc.: Current Employment; Holds F. Hoffmann-La Roche Ltd SSARs, RSU, shares: Current equity holder in publicly-traded company. Yao:Genentech, Inc.: Current Employment.
Mosunetuzumab (Lunsumio) is a bispecific CD20-directed CD3 T-cell engager indicated for the treatment of adult patients with relapsed or refractory FL after two or more lines of systemic therapy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal