Introduction
The introduction of TKI in CP-CML enabled responders to reach a similar survival to that of the general population. However, recent small-sized real-life studies showed that 1 in 5 CP-CML patients (pts) received at least 3 lines of TKI, mainly due to resistance or the onset of adverse events (AE). The objective of this study is to document real-life TKI treatment patterns nationwide in CML pts with a long-term follow-up, with a focus on pts who initiated at least a 3 rd line and associated disease burden.
Methods
This real-life non-interventional study was conducted using the French nationwide claim database (SNDS, Système National des Données de Santé), covering over 99% of French beneficiaries. Pts who initiated their first TKI with an indication for CML (imatinib, dasatinib, nilotinib, bosutinib, ponatinib) between January 2010 and December 2018, aged 18 years old or older at TKI initiation (index date T 0), and with healthcare resource use (HCRU) markers of CML - i.e., reasons for hospitalization and reported Long-Term Disease - without markers of confounding disease for the use of TKIs, were included. Treatment patterns were assessed from T 0 to December 2020 or death, through the description of TKI treatment sequences (TKI lines), adherence - discontinuations (≥ 3 months) during a TKI line and Medication Possession Ratio (MPR) -, and line duration (Time to Next Treatment (TTNT) estimates). TTNT was calculated as cumulative incidence of TKI switches, with death as competing risk; switches were defined as discontinuation of a TKI followed by the start of a different TKI.
Results
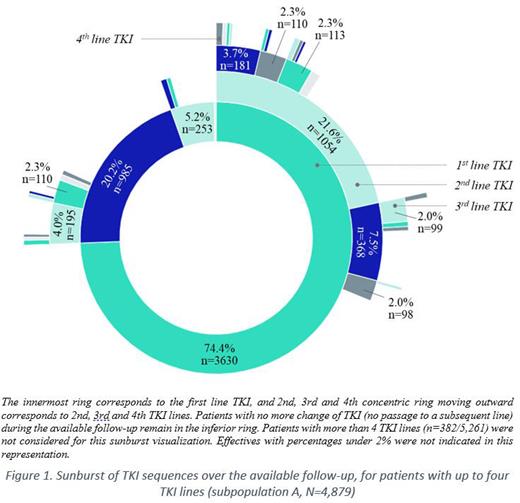
A total of 5,261 CML pts initiated their 1 st line TKI, with a median age at T 0 of 59.5 years (IQR: 23.2) and a M/F ratio of 1.2. The median follow-up from T 0 was 5.6 years (IQR: 4.6). Respectively 2,299, 1,017 and 426 pts initiated 2 nd, 3 rd and 4 th TKI line. Most frequent TKIs used as 1 st and 2 nd lines were imatinib (73.1%), and dasatinib (60.4%; Figure 1), respectively. From the 3 rd line, TKI patterns changed following the reimbursement of bosutinib in 2015 and ponatinib in 2016, and subsequently stabilized; shares in pts treated in 3 rd line and more at the beginning of 2020 were roughly equally distributed between marketed TKIs.
TKI discontinuations rates during a line varied from 76.2% for 1 st line to 56.2% for 4 th lines. The median cumulative duration of discontinuations tended to decrease across lines (2.9, 2.5, 2.3 and 2.1 months for 1 st, 2 nd, 3 rd, and 4 th line, respectively); IQRs across lines were similar. The proportion of pts with a good medication adherence (MPR ≥ 80%) was around 80% for all TKI lines.
TTNT was similar across all TKIs and all lines; 10-year TTNT rates were 48% (95CI: [47%; 50%]), 56% [53%; 59%], 56% [51%; 61%], 48% [42%; 54%] for 1 st, 2 nd, 3 rd, and 4 th lines. For pts who switched, most of switches occurred during the first year following line initiation (51% of switches observed in the first year for both 1 st, 2 nd, 3 rd, and 4 th lines), 24% occurred during the second year, and 10% during the third year. The 10-year cumulative incidences of death were 12% (95CI: [11%; 14%]), 9% [8%; 11%], 12% [9%; 16%], 14% [6%; 25%] for 1 st, 2 nd, 3 rd, and 4 th lines, respectively.
The proportion of CML pts who initiated at least 3 lines of TKIs was 19.3% from raw estimates (n=1,017/5,261) and would be 27% based on TTNT estimates. Their characteristics were similar to pts who initiated 1 st line TKI (sociodemographic, comorbidities). Regarding HCRU, respectively 37.2% and 29.8% of pts had at least one hospitalization during 1 st and 6 th semesters following 3 rd line initiation; 7.9% to 5.8% visited emergency room, and around 10% had at least one sick leave per semester.
Conclusion
Using one of the largest cohorts of CML pts ever studied, with a follow-up period up to 10 years (median 5.6 years), this real-life study confirmed that multiple TKI switches are now common. We confirmed that at least 1 out of 5 CML patient fails at least two TKI lines. From TTNT estimates, surrogate marker for the duration of clinical benefit, lines of TKI would be effective over the long-term for around half of pts, and half of the switches occur within the first two years, regardless of line position. Finally, this real-world study illustrates the need for permanent care of these pts and alternative treatment options to avoid resistance or intolerance and induce a similar survival as the general population of the same age.
Disclosures
Benmerad:Novartis: Other: service provider as a Contract Research Organization. Chassetuillier:Novartis: Other: service provider as a Contract Research Organization. Lecoq:Novartis: Current Employment. Favier:Novartis: Current Employment. Trancart:Novartis: Current Employment. Lajoinie:Novartis: Other: service provider as a Contract Research Organization. Nicolini:SUN pharma: Honoraria, Membership on an entity's Board of Directors or advisory committees; INCYTE BIOSCIENCES: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal