Background: Ide-cel and cilta-cel received regulatory approval for the treatment of patients with relapsed or refractory multiple myeloma (MM) after ≥4 prior lines of therapy, and almost all eligible patients in the US and Europe have previously received at least one autologous hematopoietic cell transplant (autoHCT). Even for those who received upfront autoHCT, salvage transplant (either second autoHCT or allogeneic HCT) for patients with relapsed MM was shown to be effective and feasible. Therefore, comparing CAR-T cell therapy with salvage transplant could facilitate future selection of patients with relapsed MM, as eligibility for transplant (especially allogeneic HCT) differs significantly from that of CAR-T.
Methods: We leveraged a large European dataset to identify relapsed MM patients who received either salvage autoHCT, allogeneic HCT, or CAR-T cell therapy. To minimize selection bias, we only included patients who received salvage treatment from 2018 onwards and who had documented time of relapse. Furthermore, to control for differences in patient characteristics, we applied propensity score (PS) matching for the comparison: CAR-T versus auto HCT and versus allogeneic HCT.
We performed individual matching with the “nearest neighbour” method, using a caliper=0.2. The model for PS estimation included patient sex, MM classification at diagnosis, previous transplant history (single or tandem auto), interval between upfront transplant and relapse and between relapse and CAR-T/salvage transplant, age, performance status and disease status at CAR-T/salvage transplant. Endpoints of matched groups were compared by Log-Rank test or Gray test stratified on the pair.
Primary endpoint was progression-free survival (PFS) at 1 year with 95% confidence interval (CI). Secondary endpoints constituted non-relapse mortality, incidence of relapse, and overall survival (OS).
Results: We included 4292 patients, of whom 3858 received salvage autoHCT, 371 allogeneic HCT, and 63 CAR-T cell therapy (60% ide-cel, 33% cilta-cel, 7% other investigational). Median age at time of salvage treatment was 62 years for autoHCT, 54 years for allogeneic HCT, and 61 years for CAR-T cell therapy (P<0.001). More than 60% of patients were male in all groups, and IgG subtype of MM was present in 52% of autoHCT, 48% of allogeneic HCT, and 44% of CAR-T cell therapy. More patients receiving CAR-T cell therapy had a Karnofsky performance status <80% (41%) versus 32% of autoHCT and 29% of allogeneic HCT.
In terms of response, complete response to treatment was observed in 40% for autoHCT, 63% for allogeneic HCT, and 44% for CAR-T cell therapy. Partial remission or very good partial remission was achieved 11%, 9%, and 35%, respectively (P<0.001).
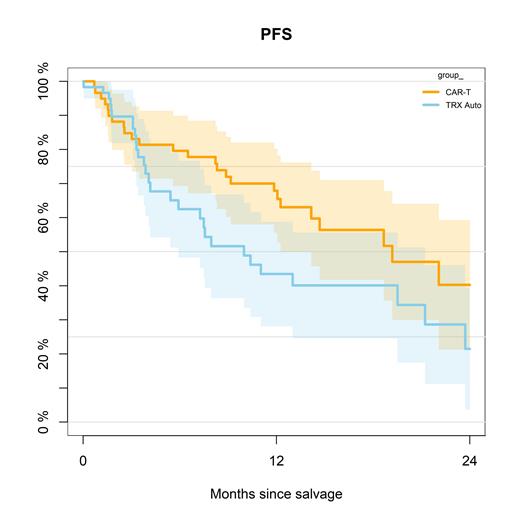
Median follow-up was 16.7 months for the entire cohort. The PS method could successfully match almost all patients for the comparison of CAR-T cell therapy versus salvage autoHCT (59 patient, respectively). The 1-year PFS for CAR-T cell therapy versus autoHCT was 68% (95% CI, 56-80%) versus 44% (95% CI, 28-59%; P=0.048; Figure 1). The 1-year non-relapse mortality was 9% (95% CI, 2-17%) versus 0% (P=0.31). Cumulative incidence of relapse at 1 year was 23% versus 57% (P=0.028), and 1-year overall survival was 81% versus 68% (P=0.059).
The PS method matched 33 patients for the comparison of CAR-T cell therapy versus salvage allogeneic HCT. The 1-year PFS was 72% (95% CI, 56-88%) versus 39% (95% CI, 18-60%; P=0.346). Non-relapse mortality at 1-year was 13% (95% CI, 1-25%) versus 23% (95% CI, 7-43%; P=0.257). The 1-year cumulative incidence of relapse was 15% versus 36% (P=0.763), and the 1-year overall survival was 84% (95% CI, 71-97%) versus 60% (95% CI, 39-81%; P=0.083).
Conclusion: This multicenter retrospective study compared CAR-T cell therapy with salvage transplantation strategies for relapsed MM. CAR- T cell therapy appeared to improve outcomes, especially in comparison with salvage autoHCT. The higher absolute rates of non-relapse mortality observed with CAR-T cell therapy may not counterbalance overall beneficial results, and rather highlights the need for careful selection of patients. Longer follow-up will be needed for definitive results.
Disclosures
Schönland:Janssen, Prothena, Celgene, Binding Site, Jazz: Other: Travel grant; Janssen, Takeda, Pfizer, Prothena: Honoraria; Prothena, Janssen, Sanofi: Research Funding. Roeloffzen:Janssen: Other: Travel grants, honoraria or advisory board (not personal); Bristol Myers Squibb: Other: Travel grants, honoraria or advisory board (not personal); AbbVie: Other: Travel grants, honoraria or advisory board (not personal); Sanofi: Other: Travel grants, honoraria or advisory board (not personal); Amgen: Other: Travel grants, honoraria or advisory board (not personal). Wulf:Novartis: Honoraria, Speakers Bureau; Gilead: Honoraria, Speakers Bureau. Mielke:SWECARNET: Other: Founder/Leadership (via my institution) ; ScientifyResearch: Other: Founder (spouse) ; Immunicum/Mendes, Miltenyi: Other: Participation on a Data Safety Monitoring Board or Advisory Board; Celgene/BMS, Novartis, Janssen, Gilead/KITE, JSMO, Pfizer: Speakers Bureau. McLornan:Abbvie: Honoraria; Jazz Pharma: Honoraria; Novartis: Honoraria; UK ALL RIC TRIAL - DSM board: Other: participation on a data safety monitoring board or advisory board; EBMT Scientific Council Member: Other: Chair of EBMT CMWP; Imago Biosciences: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal