Background: Hypophosphatemia is a frequent side-effect of certain intravenous (i.v.) iron formulations, and a recent meta-analysis of prospective clinical trials reported that 54% of patients treated with ferric carboxymaltose (FCM) develop hypophosphatemia. While the incidence and time-course of hypophosphatemia are well known, the frequency of clinical consequences of these changes is poorly described. FCM infusions cause high intact fibroblast growth factor 23 (iFGF23), which is associated with hypophosphatemia, hyperphosphaturia, hypocalcitriolemia, hypocalcemia, secondary hypoparathyroidism, which is summarized as the ‘6H-syndrome’ that can ultimately result in osteomalacia. The association between treatment with FCM, high iFGF23, osteomalacia with fractures and nephrolithiasis is well documented in multiple case reports. This is not the case for ferric derisomaltose (FDI).
Aims: The aim of the present study was to assess the incidence of fractures or radiological signs of osteomalacia and kidney stone in patients treated with FCM or FDI.
Methods: Patients treated with FCM or FDI between January 2010 und December 2019 were identified by retrospective assessment of electronic health records of the University Hospital in Innsbruck, which is a large tertiary referral center in Western Austria. Dose, time of infusion, comorbidities and haematological parameters, serum iron parameters as well as markers of mineral metabolism were extracted from the healthcare records of each patient. Radiological and clinical endpoints included fractures, radiological signs of osteomalacia and kidney stone disease after the first infusion of FCM or FDI. The year before the first infusion was used as a control period.
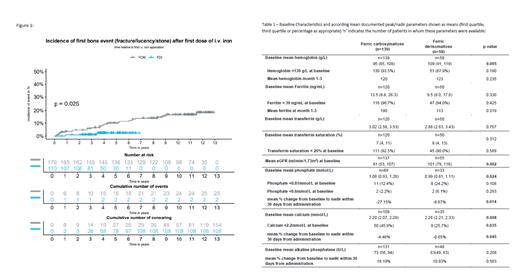
Results: In total, 289 patients were included and the median follow-up time was 5.8 years. Mean age was 44.6 years and 184 of 289 patients (64%) were females. In total 179 patients were treated with FCM and 110 patients received FDI. Grouping patients by drug revealed no differences in cumulative iron dose or number of drug applications. At baseline, serum creatinine was significantly lower in FDI-treated patients, but within the normal range for both groups. Mean hemoglobin concentrations were significantly lower in FCM-treated than in FDI-treated patients (95 g/L vs. 109 g/L) with no statistically significant differences in mean ferritin concentrations between groups at baseline. Assessment of treatment response 1-3 months after i.v. iron treatment showed a robust increase in hemoglobin and serum ferritin in both treatment groups. Baseline phosphate was significantly lower in FDI-treated patients with 3.07 mg/dL versus 3.34 mg/dL in the FCM treatment group. In FCM-treated patients a significantly greater change from baseline to 1-3 months after administration was found for phosphorous and calcium (Table 1).
Hypophosphatemia was more commonly documented and more severe in FCM-treated patients, who also showed a significant increase in ALP. In a time to event analysis (fracture, osteomalacia and kidney stones) a significantly higher proportion of patients reached the combined endpoint in the FCM treatment group than in FDI-treated patients (Figure 1). Univariate Cox-regression for fractures after the infusion showed a HR of 4.54 for FCM treatment when compared to FDI (p=0.04). In contrast, no difference in fracture incidence was present in the year before the first iron infusion when both treatment groups were compared.
Conclusions: In this retrospective analysis the combined event rate of fracture, radiological signs of osteomalacia and kidney stones was significantly higher among patients treated with FCM than after FDI treatment. This finding suggests that the observed changes in markers of mineral metabolism could indicate that FCM treatment is associated with a significantly higher risk of clinically relevant bone complications and kidney stone disease.
Disclosures
Zoller:Pharmacosmos A/S: Honoraria, Research Funding; CSL Vifor: Honoraria; Pierre Fabre Group: Honoraria; Medice Arzneimittel GmbH: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal