Background: Marstacimab (PF-06741086) targets tissue factor pathway inhibitor (TFPI) to restore hemostasis via the extrinsic pathway of blood coagulation. In phase 1/2 studies, long-term subcutaneous (SC) administration of marstacimab reduced bleeding episodes in adults with severe hemophilia A (HA) or hemophilia B (HB) (factor VIII or factor IX [FIX] ˂1%, respectively), with or without inhibitors. BASIS, the pivotal phase 3 study of marstacimab in adolescents and adults with severe HA (<1%) or moderately severe to severe (FIX ≤2%) HB, is ongoing. Given the potential risk for thrombotic and thromboembolic complications with novel non-factor-based agents, integrated safety analysis of studies of such agents are of interest. Wereport an integrated analysis of marstacimab safety in participants with HA or HB.
Methods: Data from participants in the phase 1b/2 study (NCT02974855), its long-term follow-up (LTFU; NCT03363321), the pivotal phase 3 BASIS study (NCT03938792), and its long-term extension (LTE; NCT05145127) were included. In the phase 1b/2 study, male participants (N=26) with severe HA or HB, with or without inhibitors, were assigned to escalating weekly SC doses of marstacimab based on inhibitor status (without inhibitors: 300 mg, a single 300-mg loading dose with subsequent 150-mg doses, or 450 mg; with inhibitors: 300 mg). In the BASIS study, male participants (N=163) with severe HA or moderately severe to severe HB, with or without inhibitors, received a single SC 300-mg loading dose followed by once-weekly 150 mg in the 12-month active treatment phase (ATP). Adverse events (AEs), serious adverse events (SAEs), and AEs of special interest (AESI), including thromboembolic events and injection site reactions (ISRs), were monitored.
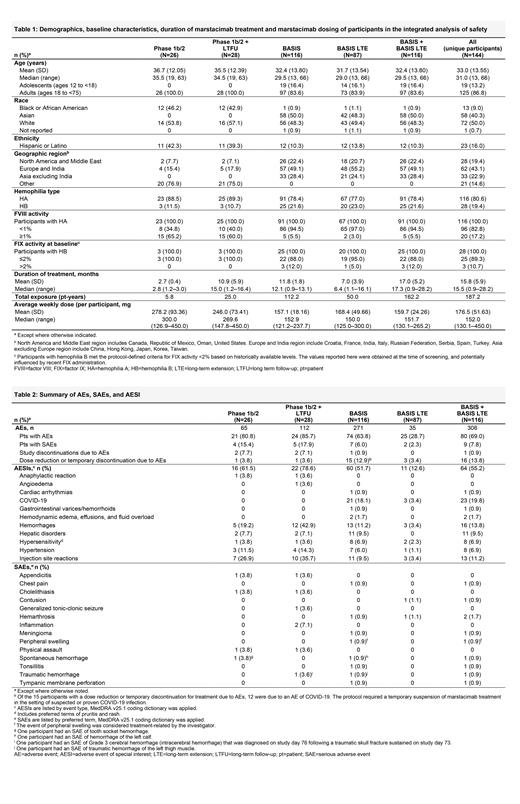
Results: Overall, 144 unique participants received marstacimab and were included in this analysis: 28 in the phase 1b/2 study (median treatment duration, 2.8 [range, 1.2-3.0] months) and its LTFU (15.0 [1.2-16.4] months) and 116 in the BASIS study (12.1 [0.9-13.1] months) and its LTE (6.4 [1.1-16.1] months; overall BASIS + LTE, 17.3 [0.9-28.2] months). Demographics, baseline characteristics, and marstacimab treatment and dosing ( Table 1) and a summary of safety are shown in Table 2. There were no treatment-related SAEs in the phase 1b/2 study and 1 treatment-related SAE (peripheral swelling) in the BASIS study and its LTE. There were no deaths in any study. There were no AEs related to thromboembolism during treatment in participants with HA or HB across the marstacimab clinical program. Adverse drug reactions were consistent across studies. In the BASIS study and its LTE, ISRs (n=13, 11.2%) tended to be transient and mild in severity and did not lead to discontinuation from the study: other reactions were also mild: pruritus (n=4, 3.4%); rash (n=1, 0.9%) and headache (n=8, 6.9%). One participant, with a history of hemorrhoids, in the BASIS ATP had a grade 2 event of thrombosed hemorrhoids considered related to marstacimab (a local inflammation event, not related to any other thromboembolic event; dose was not interrupted or changed due to this event). No clinically important findings in laboratory values were associated with marstacimab. In the BASIS ATP, antidrug antibodies (ADAs) developed in 23/112 participants (20.5% incidence; titers were low and resolved in 22/23 participants by end of study); 6/23 were positive for neutralizing antibodies (NAbs; titers were transient and resolved by the end of study). In the LTE, ADA developed in 1/44 (2.3%) participants; the ADA titer was negative prior to marstacimab but positive at the end of the main study (titer = 2.48), positive at Day 180 in the LTE (titer = 2.41), and the participant tested negative for NAbs at both timepoints. No differences in the marstacimab safety profile, as determined by AEs, SAEs, AESIs, and discontinuations due to AEs, were reported in ADA positive or negative participants. Across the BASIS study and its LTE, 18 participants had a marstacimab dose escalation from 150 mg to 300 mg; 8/18 (44.4%) had AEs, all mild or moderate in severity and there were no AEs or SAEs leading to treatment discontinuation.
Conclusion: Once-weekly SC marstacimab was well tolerated in participants with severe HA or moderately severe to severe HB, without inhibitors, with a low rate and mild severity of ISRs, transient ADAs, and no thromboembolic events with continuous treatment up to 28 months in the phase 3 program.
OffLabel Disclosure:
Acharya:Bayer: Honoraria; Pfizer: Honoraria. Mahlangu:Sandoz: Research Funding; Pfizer: Research Funding; Spark Therapeutics: Research Funding; Roche: Research Funding; Sanofi: Research Funding; BioMarin Pharmaceutical Inc.: Research Funding; Novo Nordisk: Research Funding; Catalyst: Research Funding. Taylor:Pfizer: Current Employment, Current equity holder in publicly-traded company. Hwang:Pfizer: Current Employment, Current equity holder in publicly-traded company. Hinnershitz:Pfizer: Current Employment, Current equity holder in publicly-traded company. Mefyod:Pfizer: Current Employment, Current equity holder in publicly-traded company. Palladino:Pfizer: Current Employment, Current equity holder in publicly-traded company. Biondo:Pfizer Inc: Current Employment, Current equity holder in publicly-traded company. Gould:Pfizer: Current Employment, Current equity holder in publicly-traded company. Teeter:Pfizer: Current Employment, Current equity holder in publicly-traded company.
Marstacimab (PF-06741086) is a monoclonal antibody targeted to the tissue factor pathway inhibitor (TFPI) protein to improve hemostasis via the extrinsic pathway of blood coagulation. Marstacimab is currently in development for the treatment of hemophilia A and hemophilia B, with or without inhibitors.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal