Long noncoding RNAs (lncRNAs), defined as the transcripts longer than 200 nt without protein-coding capacity, have been found to be aberrantly expressed in diverse human diseases including leukemia. Our previous study identified Familial acute myelogenous leukemia related factor ( FAMLF) in an acute myeloblastic leukemia (AML) pedigree. Two splice variants of FAMLF, FAMLF-1 and FAMLF-2 were detected. FAMLF-1 is upgraded in AML patients and associated with poor prognosis. However, the full cDNA sequence of FAMLF-2 is not verified and its role in leukemogenesis remain unclear. Herein, we aim to investigate the functional and mechanistic roles of FAMLF-2 in leukemia.
Using real-time quantitative RT-PCR assay, we found that the expression of FAMLF-2 was at higher levels in Acute Lymphoblastic Leukemia(ALL) cell lines when compared to AML cell lines. Bone marrow cells were obtained from de novo ALL patients. qRT-PCR showed FAMLF-2 was up-regulated in ALL patients (N=115) compared to healthy controls (N=72). The FAMLF-2 expression was positively correlated with the poor prognosis in ALL patients by Kaplan-Meier analysis. These data demonstrated that FAMLF-2 is overexpressed in ALL patients and is associated with poor prognosis. To verify the full cDNA sequence of FAMLF-2, 5‘ and 3‘ rapid amplification of the cDNA ends (RACE) was performed, and we identified the 2393 nt of FAMLF-2. FAMLF-2 is predicted to not have protein-coding potential. Further subcellular analysis showed FAMLF-2 is mainly localized in the nucleus of Jurkat cells by lncRNA FISH and subcellular fractionation assay.
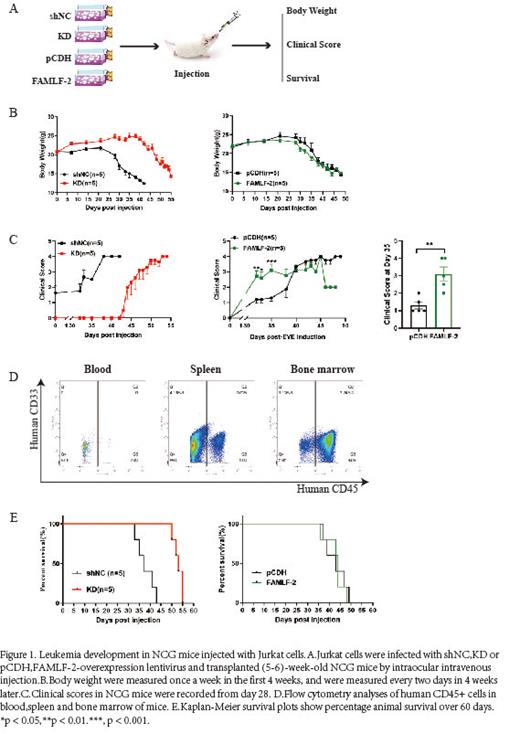
To further verify the role of FAMLF-2 in ALL leukemogenesis, a xenograft model was established in NCG mice by subcutaneously or intravenously injection of engineered Jurkat cells (knockdown group: shNC and sh FAMLF-2, overexpression group: pCDH and FAMLF-2). We found that the size and weight of tumors in FAMLF-2 knockdown group were significantly decreased compared with the empty vector in subcutaneous ALL xenograft tumor model. and the mice in the sh FAMLF-2 groups survived longer than those in the shNC groups in introvenous ALL xenograft tumor model (sh FAMLF-2 vs. shNC: 55 days vs. 43 days, P<0.01). These data suggested that downregulation of FAMLF-2 inhibits leukemia development. Although no significant difference was found in the overall survival of mice overexpressed FAMLF-2. We observed the accelerated central nervous system(CNS) infiltration of leukemia cells when FAMLF-2 was overexpressed (clinical score at 32th day from Vector vs. FAMLF-2: 2.7 scores vs. 1.2 scores, p < 0.01 ). In vitro study revealed knockdown of FAMLF-2 inhibited cell proliferation and promoted cell apoptosis in ALL cells, and vice versa. Taken together, our clinical, mouse model, and AML cell data demonstrated that FAMLF-2 promotes leukemogenesis.
RNA sequencing was performed to study how FAMLF-2 promotes ALL development. Gene Set Enrichment Analysis (GSEA) of genes downregulated by FAMLF-2 showed “Myc targets” with the highest normalised enrichment score (NES) in Jurkat cells. C-Myc is a well-recognized transcription factor that involves in leukemogenesis. To investigate whether FAMLF-2 performed direct interaction with c-Myc, we performed RNA pull-down followed by mass spectrometry analysis. Instead of c-Myc, DExH-Box Helicase 9 (DHX9) has the highest abundance. The Western blot and RNA immunoprecipitation (RIP) assay were further performed to validated the direct interaction between FAMLF-2 and DHX9. DHX9 is an RNA helicase that participates in c-Myc mRNA stability. Inhibition of FAMLF-2 suppressed DHX9 and c-Myc expression. Reduced interaction between DHX9 protein and c-Myc mRNA were observed when FAMLF-2 were downregulated. Furthermore, RNA stability assays suggested that knockdown of FAMLF-2 leads to a declined half-life of c-Myc mRNA. Collectively, these results demonstrated that FAMLF-2 regulates the stability of c-Myc mRNA through direct binding of DHX9, thus involved in the ALL development.
In summary, our study revealed the previous undefined cDNA sequence of FAMLF-2, and provides new insights of FAMLF-2 in leukemogenesis via the DHX9/c-Myc axis, which might act as a prospective prognostic biological marker in ALL.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal