Introduction
Chronic Lymphocytic Leukemia (CLL) is an indolent B-cell lymphoproliferative disease that carries the risk of evolving into a life-threatening aggressive histology, known as Richter's syndrome. Despite the advent of new agents such as ibrutinib and venetoclax that have dramatically changed the clinical fate of CLL, young patients still experience refractory and resistant disease, with few therapeutic options available over time.
Therefore, the need for new strategies is pivotal for this chronic and insidious condition.
Drug resistance might be triggered by the tumor microenvironment (TME), which provides survival signals to CLL tumor cells. Their interaction is mainly established with monocyte-derived nurse-like cells (NLCs). NLCs resemble the immunosuppressive M2-like tumor-associated macrophages (TAMs) and can create a profitable connection with CLL in terms of increasing proliferation and survival (Filip, Blood Cells Mol Dis. 2013).
The possibility of targeting the TME by blocking the “Don't Eat Me” signal (DEMs) CD47 has provided restoration of phagocytosis within TME and interesting clinical results in heavily pretreated cases of lymphoma (Advani, NEJM. 2019). The use of agents targeting the DEMs within TME could be a promising approach in CLL as well.
CD24 is another DEMs that was first validated in a preclinical model of solid cancer (Barkal, Nature. 2019). It was subsequently found to be upregulated in Mantle-cell lymphoma (MCL) and involved in DEMs as well, since CD24 blockade provided an increase in phagocytosis of MCL cells in vitro (Aroldi, Blood. 2021). This effect was particularly evident in association with anti-CD47 monoclonal antibody (mAb) and Rituximab (Aroldi, Blood. 2022).
Although MCL has different pathogenesis compared to CLL, these two entities share some features, particularly regarding the B-cell of origin and the vital interaction with non-cancerous cells within TME. In this study, we explore the possibility of targeting CD24 in human primary samples of CLL (huCLL), redirecting the activity of NLCs against CLL cells using anti-CD24 mAb alone or in combination with other agents (anti-CD47, Rituximab).
Methods
We analyzed a microarray dataset of patients with CLL to check for any correlation between CD24 expression and survival (GSE22762). We also checked the CD24 antigen expression of huCLL, isolated and differentiated monocytes into M2-like TAMs in vitro, as well as conducted co-culture assays using flow cytometry. These methods were previously described (Aroldi, Blood. 2021; Aroldi, Blood. 2022). Unstimulated autologous CD14+ cell population - defined as precursors of NLCs - were isolated from patients with CLL and used for co-culture analysis without any previous cytokine stimulation for differentiation.
Results
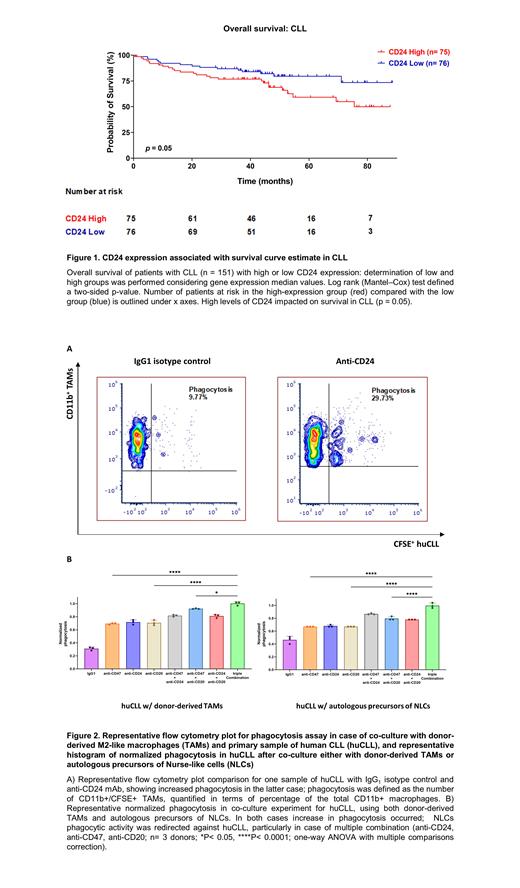
In a cohort of CLL patients, higher CD24 levels correlated with a lower survival rate compared to lower expression ones (Figure 1). We also checked the CD24 surface in an extended cohort of huCLL and compared it to MEC-1 and PCL-12. huCLL showed higher CD24 surface levels than MEC-1 and PCL-12 and demonstrated better phagocytosis when co-cultured with M2-like TAMs and anti-CD24 mAb (Figure 2A). We then isolated precursors of circulating NLCs from 5 huCLL patients and found higher levels of Siglec-10 (CD24 ligand) than in healthy controls. In this setting, blocking CD24 redirected the phagocytic activity of NLCs against huCLL, showing higher phagocytic rate in cases of multiple combinations (anti-CD24, anti-CD47, anti-CD20, Figure 2B).
Conclusions
CLL could be another blood cancer subtype sensitive to CD24/Siglec-10 DEMs blocking agents, and CD24 expression negatively impacts survival. Anti-CD24 mAb, in particular, showed the ability to redirect patient-derived NLCs' phagocytic activity to cognate huCLL cancer cells, resulting in higher clearance in cases of multiple combination. Boosting the autologous innate immune system might, therefore, provide further therapeutic options in the setting of CD24 + CLL, particularly for those patients who have failed several lines of treatment and have limited options available.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal