Background: The impact of copy number alterations (CNAs) or mutations on outcomes has been broadly analyzed in pediatric acute lymphoblastic leukemia (ALL). However, the magnitude of the effect was often marginal, frequently restricted to specific genetic subtypes and rarely replicated across independent cohorts in adult ALL.
Aim: We tried to analyze the prognostic relevance of genetic copy number alterations and gene mutations in patients with Ph-negative ALL who were treated with modified hyper-CVAD based chemotherapy followed by allogeneic hematopoietic cell transplantation (alllo-HCT).
Methods: We investigated the role of CNAs or mutations to refine risk stratification in 128 adults with newly diagnosed Philadelphia chromosome (Ph)-negative B-cell precursor ALL treated with intensive chemotherapy followed by allo-HCT for post-remission therapy. We performed multiplex ligation-dependent probe amplification (MLPA) to detect deletions of 11 genes ( IKZF1, CDKN2A/B, EBF1, ETV6, PAX5, BTG1, JAK2, RB1, PAR1, ZFY) and high throughput sequencing (HTS) of 70 gene mutations.
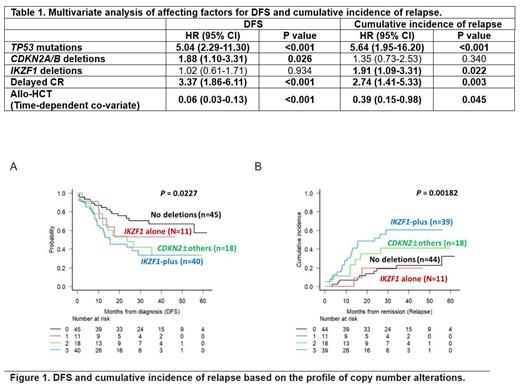
Results: MLPA analysis showed that IKZF1 and CDKN2A/B deletions were observed in 54 (42.2%) and 52 (40.6%) patients, respectively. The remaining gene deletions were: PAX5 in 29 (22.6%), ETV6 in 22 (17.2%), BTG1 in 15 (11.7%), EBF1 in 14 (10.9%), RB1 in 13 (10.1%), JAK2 in 8 (6.2%), and PAR1 in 4 (3.1%). By HTS, NRAS (n=21, 16.4%), KRAS (n=11, 8.6%), TP53 (n=14, 10.9%), and PTPN11 (n=12, 9.4%) mutations were frequently detected. Overall, multivariate analysis for disease-free survival (DFS) and cumulative incidence of relapse (Table 1) showed that TP53 mutations (HR 5.04, 95%CI 2.29-11.30, P<0.001) and CDKN2A/B deletions (HR 1.88, 95%CI 1.10-3.31, P=0.026) were significantly associated with a poorer DFS. Patients with TP53 mutations (HR 5.64, 95%CI 1.95-16.20, P<0.001) and IKZF1 deletions (HR 1.91, 95%CI 1.09-3.31, P=0.022) had a higher relapse risk. To better define the prognostic role of CNAs, further analyses were restricted to patients excluding 14 TP53 mutations (n=114). Here, we classified patients into 4 subgroups; (1) No deletions (n=45, 39.5%), (2) IKZF1 deletion-alone (n=11, 9.6%), (3) IKZF1plus (n=40, 35.1%), and (4) CDKN2A/B±other deletions without IKZF1 deletions (n=18, 15.8%). Patients with IKZF1plus or CDKN2A/B±other deletions had an inferior DFS ( P=0.023) and higher relapse incidence ( P=0.002) than patients having no deletions. The results were similarly reproduced in patients receiving allo-HCT in first complete remission.
Conclusions: Our data showed that IKZF1 or CDKN2A/B deletions and TP53 mutations may confer a poor prognosis for adult Ph-negative B-cell precursor ALL in the setting of intensive chemotherapy and allo-HCT. The combination of genomic abnormalities and minimal residual disease response may further refine risk stratification and better select patients who could benefit from novel therapeutic approaches.
Disclosures
Lee:Kira: Consultancy; Achillion: Research Funding; Arrowhead: Consultancy; AlloVir: Consultancy; Alexion, AstraZeneca Rare Disease: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Samsung: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal