Background
Peripheral T-cell Lymphoma (PTCL) affects a diverse cohort of patients for which survival disparities are evident in minorities. Clinical trial enrollment does not match the demographic diversity represented in PTCL and minorities are historically underrepresented. In this single-center retrospective analysis, we aim to answer if clinical trial enrollment can close the gap in survival outcomes in minorities compared to non-minorities in the modern era.
Methods
After IRB approval, 225 patients with PTCL were identified between 1991-2022, of which 199 had sufficient data for review. Medical records were reviewed for baseline characteristics, treatment parameters, and demographic data. Income quintile was calculated for the cohort using zip code level census data. The social deprivation index ( SDI) was calculated as a composite measure of demographic characteristics in the 2015-2019 American Community Survey. Overall survival ( OS) was calculated from diagnosis to time of death or last follow-up. Kaplan-Meier method was used to estimate survival probability. Survival difference was tested by log-rank and Cox regression complete case analysis. Due to the small sample size, Black and Hispanic patients were combined ( minority) and White with Asian/unknown/other ( non-minority) to delineate differences between groups. The OS was compared across four groups: ( 1) minority with first-line clinical trial, ( 2) minority without first-line clinical trial, ( 3) non-minority with first-line clinical trial, and ( 4) and non-minority without first-line clinical trial.
Results
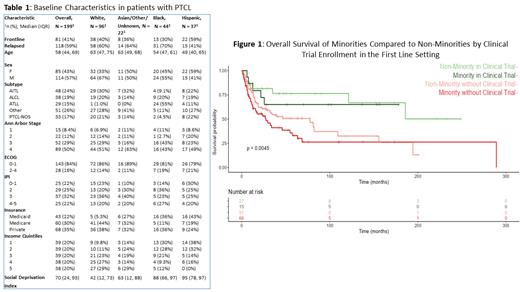
From the initial cohort of 199 patients, 118 (59%) had relapsed/refractory (R/R) disease. The median age of diagnosis was 58 years. A total of 48.2% of patients were White (n=96), 22.1% Black (n=44), 18.5% Hispanic (n=37), and 11.1% Asian/other/unknown (n=22). The major PTCL subtypes included AITL 24% (n=48), ALCL 19% (n=38), PTCL_NOS 17% (n=33), and ATLL 15% (n=29). Importantly, 55% (n=24) of the black patient population had ATLL. Baseline characteristics are shown in Table 1. In total, 27% (n=53) were exposed to a novel agent in the first-line, and 64% (n=76) in second-line. Clinical trial enrollment accounted for 21% (n=42) first-line, and 34% (n=40) second-line. Black and Hispanic patients were more likely to be in the lower income quintile, 30% and 38%, respectively. The SDI score (0-100; higher score indicates more deprivation) was higher for Black (88), and Hispanic (95) patients compared to White (42) and Asian/other/unknown (63). However, SDI score was not associated with survival in cox models.
In the first-line setting, there was a survival difference for both minorities and non-minorities enrolled in a clinical trial compared to those who were not ( Figure 1, p=0.0045), but there was no difference for novel agents (p=0.18).Given the aggressive nature of ATLL and differences in baseline covariates, a cox model was used to compare the four groups and OS was analyzed controlling for age, sex, insurance, stage, ECOG, and ATLL. Minorities performed similarly to non-minorities when participating in a clinical trial ( HR: 1.19 95% CI: [0.32, 4.4]), while minorities and non-minorities not enrolled on a clinical trial performed worse overall ( HR: 1.97, 95% CI: [0.77, 5.0], and HR: 1.49, 95% CI: [0.64, 3.4], respectively).
In the second-line setting, both minorities and non-minorities appeared to experience OS benefit favoring clinical trials compared to those without (p=0.026).
Conclusion
Black and Hispanic patients have inferior outcomes compared to Whites across most subtypes of PTCL in data published by Adams et al. over a decade ago. Our data represents a trend toward similar outcomes in minoritized compared to non-minoritized patients with clinical trial enrollment in the first-line and second-line settings. Clinical trial enrollment may close the gap in survival outcomes; however interpretation is limited by small sample size. Multi-center efforts are needed to better elucidate these findings. It is imperative to place emphasis on improved clinical trial enrollment in diverse patient populations.
*This work has been funded by ASH RTAF
Disclosures
Pro:Seattle genetics: Honoraria; Bio Secura: Honoraria. Amengual:Incyte: Consultancy; Epizyme: Honoraria; AstraZeneca: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal