Introduction: Epstein-Barr virus positive (EBV+) central nervous system lymphoproliferative diseases (CNS-LPD) are aggressive clinical conditions with poor prognosis. EBV+ CNS-LPD often occurs in the setting of immune suppression and comorbidities (solid organ transplant, autoimmunity). Treatment with high-dose methotrexate has increased survival but is not a viable option for all patients, highlighting the need for alternative treatments. Previous research has reported EBV+ CNS-LPD exhibits unique genetic and immunobiological signatures, however, few studies have reported on the epigenetic landscape. We have previously reported use of ganciclovir (GCV), zidovudine (AZT), rituximab and dexamethasone (GARD) regimen induced complete and durable responses in a cohort of 13 patients with primary CNS post-transplant lymphoproliferative disease (PCNS-PTLD). Here we present 24 patients (10 from prior cohort and 14 new) with EBV+ CNS-LPD treated with GARD. We extend follow-up time for the previous cohort and add molecular context to clinical observations. We performed DNA methylation and expression analysis of EBV viral kinases in available tumor samples and revealed unique viral epigenetic states leading to expression of antiviral drug targets in an array of EBV+ CNS-LPD.
Methods: We conducted an IRB-approved, retrospective review of patients with EBV+ CNS-LPD treated with GARD at The Ohio State University Wexner Medical Center between 1998-2022. Patient information was extracted from the electronic medical record. Treatment consisted of two-week induction of twice daily IV GCV/AZT (5mg/kg and 1,500 mg) IV dexamethasone (10-40mg), and weekly rituximab (375mg/m 2). After induction, dexamethasone was tapered, and GCV/AZT was switched to maintenance oral dosing of 450mg valganciclovir twice daily and 300mg of AZT twice daily. Rituximab was administered on days 1, 8, 15, and 22. Two-year overall survival (OS), five-year OS, and overall response rate were assessed. Available CNS-LPD biopsy samples were obtained, and expression of EBV lytic genes BZLF1, BXLF1 (thymidine kinase), and BGLF4 (protein kinase) were measured via qRT-PCR. Mass spectrometry based EpiTYPER assay was used to report the percentage of DNA CpG methylation in the gene promoters for viral BZLF1, BXLF1, BGLF4 and LMP1 genes . Biopsies from 17 patients with systemic PTLD with no CNS involvement were used for comparison.
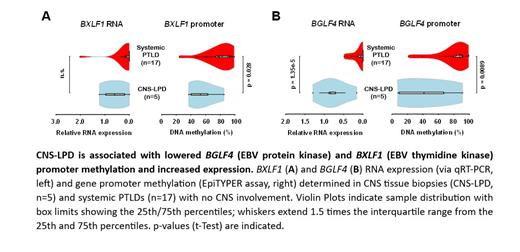
Results: 24 patients with EBV+ CNS-LPD were identified. 21 (87.5%) patients were diagnosed with a primary CNS-LPD (PCNS-LPD). Of the PCNS-LPD, 14 patients were PCNS-PTLD, 4 were receiving immunosuppression for autoimmune conditions, 1 patient had type 2 diabetes and two had no known history of immunosuppression. The remaining three patients had systemic PTLD with secondary CNS involvement. Diffuse large B-cell lymphoma was the most common histology (11; 45.8%) followed by grade 3 lymphomatoid granulomatosis (5; 20.8%), B-cell LPD (5;20.8%), polymorphic PTLD (2;8.3%) and one large cell lymphoma of ambiguous lineage. All patients were HIV negative. EBER was positive in all biopsies and EBV PCR of cerebrospinal fluid was positive in patients without biopsy. Sixteen patients (66.7%) achieved a completed response, with an overall response rate of 83%. Two-year OS was 62.5% (95% CI, 45.8-85.2%) and five-year OS was 52.8% (95% CI, 35.7%-77.9%). Five CNS-LPD brain biopsies were available for analysis. qRT-PCR of biopsy specimens showed expression of LMP1, BXLF1, and BGLF4, but not BZLF1. Expression of BGLF4 was significantly higher (p=1.35e-5) in CNS-LPD when compared to systemic EBV+ PTLD. EpiTYPER data showed significantly decreased promoter methylation for viral kinases BXLF1 and BGLF4 (p=0.0281 p=0.0089).
Conclusions: We have shown that GARD regimen shows efficacy in the treatment of EBV+ CNS-LPD, with 62.5% two-year OS and 52.8% five-year OS. Our results provide the molecular basis for expression of lytic viral kinases BXLF1 and BGLF4 in CNS-LPD by showing that both genes exhibit decreased promotor methylation. This expression of viral kinases in CNS-LPD supports the mechanistic rationale of this antiviral approach and could be further investigated as a predictive biomarker. Overall, this research highlights unique epigenetic features of EBV in CNS-LPD that support the use of antiviral, specifically the GARD regimen, in EBV+ CNS-LPD.
OffLabel Disclosure:
Dugan:Abbvie: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Sobi: Speakers Bureau; Rigel: Speakers Bureau; Bristol-Meyer-Squibb: Speakers Bureau. Haverkos:Viracta Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees. Porcu:BioGene: Membership on an entity's Board of Directors or advisory committees; Kyowa: Consultancy; Kymera: Membership on an entity's Board of Directors or advisory committees; Dren-Bio, ADCT, Lilly-Loxo, Viracta, Innate Pharma: Membership on an entity's Board of Directors or advisory committees; Ono: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Kyowa, Daiichi, Viracta, Dren Bio, Innate Pharma: Consultancy; Kyowa, Daiichi, Viracta, Dren Bio, Innate Pharma, Ono: Honoraria; Teva: Research Funding; Innate Pharma: Research Funding. Voorhees:Incyte: Research Funding; AstraZeneca: Research Funding; Novartis: Consultancy; Recordati: Consultancy, Research Funding; Morphosys: Research Funding. Baiocchi:Prelude Therapeutics: Consultancy; EUSA: Consultancy; Viracta: Membership on an entity's Board of Directors or advisory committees; Atara Biotherapeutics: Membership on an entity's Board of Directors or advisory committees.
Zidovudine (AZT) is an antiviral, nucleoside analog reverse transcriptase inhibitor, used in the treatment of HIV.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal