Introduction: High-dose methotrexate (HD-MTX) is an essential regimen for the treatment or prevention of central nervous system disease in hematologic malignancies. However, delayed MTX excretion is a major contributor to increased MTX toxicity and limits its use in populations such as younger individuals with good kidney function. Given the variable degrees of older age and poor renal function, there is a need to focus on developing a more precise predictive tool for delayed MTX excretion rather than avoiding HD-MTX treatment in all patients considered relatively high-risk. The aim of this study is to identify risk factors for delayed MTX excretion in adult patients with hematologic diseases and establish an applicable model for clinical practice.
Methods: We performed a retrospective analysis of consecutive patients with acute leukemia or malignant lymphoma who received HD-MTX therapy at Kameda Medical Center between 2011 and 2023. Standard supportive care, such as adequate hydration, urine alkalinization (pH >7.0), and leucovorin rescue, was performed according to our institutional protocol. Delayed MTX excretion was defined as plasma MTX levels ≥ 1 μmol/L at 48 h or ≥ 0.1 μmol/L 72 h after HD-MTX infusion. Univariate and multivariate analyses using logistic regression were performed to identify risk factors for delayed MTX excretion. Variables to create a nomogram was selected using a backward stepwise selection method. The internal validation of the nomogram was conducted using the bootstrap method (1000 bootstrap resample).
Results: A total of 216 patients were treated with 579 cycles (median 2, range 1-14) of HD-MTX-containing chemotherapy. The median age was 66 years (range 15-84), with male predominance (58.3%). Most patients (82.9%) had an underlying malignant lymphoma, and most cycles were administered at diagnosis (81%) and for prophylactic intent (76.4%).
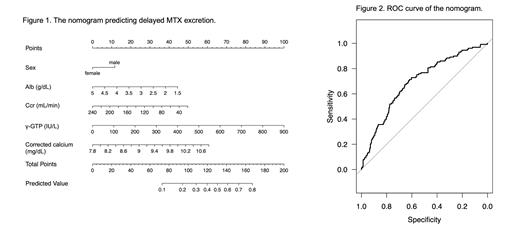
The delayed MTX excretion occurred 129 in 579 cycles (22.2%); 39 (30.2%) at 48 hours and 107 (82.9%) at 72 hours after MTX infusion, and 17 (13.2%) at both time points. On univariate analysis, neither the dose of MTX nor the dosing formula (3 hours drip or 24-hour continuous infusion) was a significant risk factor for MTX elimination failure. By contrast, older age (Odds ratio [OR] 1.022, 95% confidence interval [CI] 1.006-1.039, P =0.009), male sex (OR 1.6 [1.05-2.42], P =0.027), levels of albumin (OR 0.44 [0.302-0.639], P <0.001), creatinine clearance (Ccr) (OR 0.99 [0.985-0.998]), gamma-glutamyl transpeptidase (γ-GTP) (OR 1.004 [1.001-1.007], P =0.005), and corrected calcium (cCa) (OR 2.746 [1.646-4.651], P <0.001) significantly associated with MTX clearance. Using backward stepwise selection, sex, albumin, Ccr, γ-GTP, and cCa were selected as continuous variables to create a nomogram (Figure 1). The nomogram showed the area under the curve of 0.69 (95%CI 0.64-0.74) in predicting delayed MTX excretion (Figure 2) without significant overfitting (optimism =0.091).
Finally, we evaluated the impact of MTX elimination failure on clinical outcomes. As expected, delayed MTX excretion was highly associated with the development of any grade acute kidney injury (OR 30.9 [10.6-90.1], P <0.001). Patients who experienced a delay in MTX clearance had significantly worse survival after receiving HD-MTX than those without (5-year survival rate 38% vs. 64.5%, P <0.001). This negative effect remained significant (hazard ratio 1.73 [1.08-2.78], P =0.02) after adjusting age and disease status on multivariate analysis.
Conclusions: To our knowledge, this is the first study to construct an easy-to-use nomogram based on routinely measured biochemical data and gender predicting the risk of delayed MTX excretion. Further studies are needed to validate this model and integrate the dynamic factors such as urine output and alkalinization.
Disclosures
Matsue:AstraZeneca: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal