Background
Short interval from diagnosis (i.e., biopsy) to treatment initiation (DTI) is associated with aggressive behavior of lymphomas including diffuse large B-cell lymphoma (DLBCL), and it should be considered as an inclusion criterium in clinical trials to avoid bias given by treatment delay. However, date of biopsy (diagnosis date) could be influenced by other factors. Initial symptoms to treatment interval (STI) as well as interval between the first contact with health care system and treatmet (CTI) could have impact on survival as well as and there is no data analyzing the impact of those intervals. The aim of this analysis was to understand the correlations among these intervals and the impact on survival of patients (pts) in the real-life setting.
Methods
Altogether 1576 pts with de novo DLBCL or high-grade B-cell lymphoma from Charles University, General Hospital, Prague, Czech Republic, were identified as part of the lymphoma project (NiHiL; NCT03199066). Of them 929 pts were diagnosed between 2010-2021, and 602 (65%) pts who were treated by R-CHOP in this period entered this retrospective analysis. STI ( n = 586), CTI ( n = 535), DTI ( n = 602) were analyzed together with baseline characteristics and survival of the pts (OS, EFS).
Results
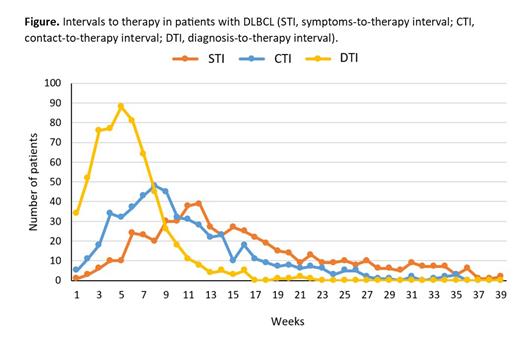
The 602 pts (median age 64 years) were diagnosed with advanced clinical stage (aCS) in 64%, extranodal (EN) involvement ≥ 2 sites in 40%, PS ECOG ≥ 2 in 26%, elevated LDH in 61%, bulky disease ≥ 7.5 cm in 41%, B-symptoms in 42%, and IPI 3-5 in 52%. Median follow-up was 7.5 years (range 0.1-13.2 years). Median STI was 15 weeks (w) (interquartile range, IQR 10-24 w), CTI 9 w (IQR 6-14 w), DTI 32 days - 5 w (IQR 3-7 w, Figure). Significant correlations among the intervals ( P <0.001 for all correlations; coefficient 0.796 for STI x CTI, 0.307 for STI x DTI, and 0.372 for CTI x DTI) were observed.
Shorter intervals to therapy, most significantly DTI, correlated with aggressive features of DLBCL, i.e., PS ECOG ≥ 2 (CTI P= 0.002, DTI P <0.001), elevated LDH (CTI P= 0.022, DTI P <0.001), B-symptoms (CTI P= 0.034, DTI P <0.001), aCS (DTI P <0.001), extranodal involvement (DTI P <0.001), IPI 3-5 (DTI P <0.001), and bulky disease (STI P= 0.005, CTI P= 0.011, DTI P <0.001).
Out of 307 pts with short DTI (≤ 32 days) we identified 183 (60%) pts with STI > 15 w; these pts didn't have different outcome in comparison to those with shorter both STI and DTI.
A pre-phase treatment ( n = 144) was associated with shorter intervals to therapy (STI P= 0.025, CTI P <0.001, DTI P <0.001) and inferior survival (EFS24 58% vs. 81%, HR 2.03, P <0.001; OS24 71% vs. 87%, HR 2.04, P <0.001).
Shorter DTI was observed in pts who didn't respond to therapy in comparison to those who responded (med. DTI 27 vs. 33 days; P= 0.038). Shorter intervals to therapy were associated with significantly inferior survival, i.e., for STI 0-7 w vs. > 7 w (EFS24 66% vs. 77%, HR 1.53, P= 0.018; OS24 75% vs. 85%, HR 1.50, P= 0.087; with no significant survival difference between groups with STI 0-15 w vs > 15 w), for CTI 0-9 w vs. > 9 w (EFS24 69% vs. 82%, HR 1.53, P= 0.003; OS24 78% vs. 90%, HR 1.64, P= 0.002), and for DTI 0-4 w vs. > 4 w (EFS24 69% vs. 82%, HR 1.53, P< 0.001; OS24 78% vs. 90%, HR 1.67, P= 0.007).
Multivariate analysis revealed all 3 intervals to be independent risk factors of survival when analyzed separately with IPI and gender (i.e., for EFS STI P= 0.039, CTI P= 0.029, DTI P= 0.028). DTI was however the only independent factor ( P= 0.001) among the 3 intervals when they were analyzed together.
Conclusion
Short intervals to therapy are independently associated with aggressive features of DLBCL and inferior survival. Despite multiple external factors that might influence the date of biopsy, DTI seems to be the most accurate predictor of survival and response to therapy. In pts with short DTI and long STI, no survival difference was observed when compared to other short-DTI pts indicating that these pts should be considered as high-risk similar to other short-DTI pts.
This work was supported by the Charles University Hematology-Oncology Cooperatio Program and grant NU21-03-00411.
Disclosures
Vodicka:Roche: Consultancy. Trněný:Takeda, BMS, Incyte, AbbVie, Amgen, F. Hoffmann-La Roche Ltd, Gilead Sciences, Janssen, MorphoSys, Novartis, Genmab, SOBI: Consultancy; Janssen, Gilead Sciences, Takeda, BMS, Amgen, AbbVie, F. Hoffmann-La Roche Ltd, MorphoSys, Novartis: Honoraria; Gilead Sciences, Takeda, BMS, F. Hoffmann-La Roche Ltd, Janssen, AbbVie: Other: Travel, Accommodation, Expenses.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal