Introduction
Recent advances in genomics in chronic myeloid leukemia (CML) patients (pts) provide new insights in understanding somatic mutation (SM) and their prognostic implications following tyrosine kinase inhibitor (TKI) therapy. DNMT3A, TET2, or ASXL1 (DTA) mutations are most frequently detected following TKI therapy and have adverse prognosis.
Five longitudinal patterns of changes in SM after TKI therapy were suggested with its clinical significance (Kim, Blood 2017): Pattern 1, persistent mutation burden despite optimal TKI response; Pattern 2, emerging mutation which correlates with TKI resistance/CML progression; Pattern 3, mutational clearance with mixed clinical outcomes; and Pattern 4 and 5 having some mutation in germline control as well as CML cells.
Clonal evolution (CE) in Philadelphia negative (Ph -) clone is found in up to 10% of CML pts who respond to TKI therapy optimally but develop +8 or -7 changes in Ph - clone. This is diagnosed only by metaphase cytogenetic test on bone marrow samples. Alternatively, fluorescence in situ hybridization (FISH) test can be used.
With recent advances in next-generation sequencing (NGS), we hypothesize that SM profiling can capture a clone carrying a SM in the Ph - population. We sequenced and analyzed mutation profiles in paired samples taken prior to TKI therapy and at longitudinal follow-up.
Patients and methods
The present study adopted single molecule-tagging and molecular inversion probe (smMIP)-based sequencing methods to analyze the DNA extracted from the mononuclear cell fraction of peripheral blood samples collected from CML pts at initial diagnosis and during follow-up. While conventional NGS has a relatively high intrinsic sequencing error rate and is limited in detecting mutations with a variant allele frequency (VAF) of less than 2%, smMIP-based sequencing overcomes this issue by tracking all sequence reads from a single strand and can detect mutations with a limit of detection up to 0.1%. The in-house CML-specific smMIP panel used in this study included 40 genes with 332 amplicon probes covering genes involved in epigenetic modifiers, activation signaling, myeloid transcription factor, spliceosome, tumor suppressor, cohesion, and miscellaneous functions.
To evaluate kinetics of mutations, we calculated doubling time of mutations. Using the mutational VAFs from 2 time points, t1 and t2, the doubling time was calculated according to the following equation: = (-2 -1) log(2) /log(-2) - log(-1), where VAF1 and VAF2 is the VAF of the mutant at time points t1 and t2, respectively.
Results
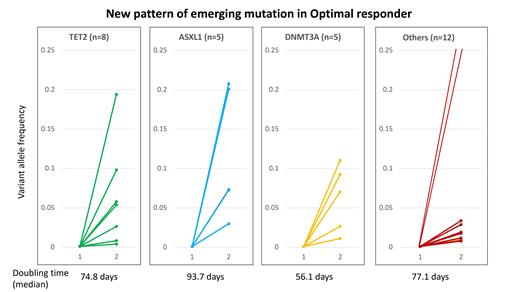
We performed targeted deep sequencing on a total of 119 serial samples from 51 CML pts (median of 405.5 days following TKI therapy). 41 pts had an optimal response, 7 were non-responders, and 3 were non-evaluable for response. Pattern 2 was observed in 1 of 7 non-responders. In 12 pts pattern 3 (mutation clearance with mixed clinical outcomes) was observed. 22 pts carried 36 mutations. At baseline 9 pts had somatic mutations. Emergence of somatic mutations in optimal responders was observed in 13 out of total 51 (25%) pts, with DNMT3A (n=5), TET2 (n=4), ASXL1 (n=4), and EZH2 (n=2). While JAK2, SF3B1, U2AF1, PHF6, TP53, BRAF, and CBL were observed in one patient each (Figure 1). Particularly, those with emerging DTA mutation were all optimal responders to TKI therapy. This pattern of mutational change is novel and has never been described before. The median doubling time was 77 days (range 21-1909 days) in the group with emergence of somatic mutation in follow-up samples. The doubling time was 56.1, 93.7, 74.8 days in DNMT3A, ASXL1, and TET2 mutations, respectively. Based on kinetics of mutations (doubling time), to capture 10-fold increase (i.e. 1 log increase) of certain mutation, NGS profile can be monitored every 6-7 months. This pattern of emergence of mutations (mostly DTA mutation) in pts responding optimally to TKI therapy is new and does not fit into the five patterns mentioned in our previous paper (Kim, Blood 2017). Thus, we propose this sixth novel pattern called “Emerging mutational clones in CML pts responding optimally to TKI therapy”.
Conclusion
We suggest a new pattern of emerging mutations in optimal responders to TKI therapy, which could derive from expansion of Ph- clone carrying some clones with emerging mutations. Longitudinal NGS monitoring in CML patients is feasible with frequency every 6-7 months.
Disclosures
Zackova:Angelini Pharma: Consultancy, Honoraria, Other: travel grant, Speakers Bureau; Pfizer: Honoraria, Other: travel grant, Speakers Bureau; Astra Zeneca: Other: travel grant; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel grant, Speakers Bureau. Kim:BMS: Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Paladin: Consultancy, Research Funding; Novartis: Consultancy, Honoraria, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal