Background:
Overactivation of the Transforming growth factor beta (TGF-β) superfamily has been associated with bone marrow failure in MDS. TGF-β binds to set of receptors that include the TGF-β receptor I kinase (also known as ALK5), that in turn phosphorylates and activates the downstream SMAD2/3 proteins. Activation of SMAD2/3 transcription factors has been shown to occur in MDS and is associated with anemia. Thus, we wanted to evaluate the preclinical efficacy of novel, clinic-ready, TGF-b receptor I kinase small molecule inhibitors IOA-359 and IOA-360 in MDS models.
Results:
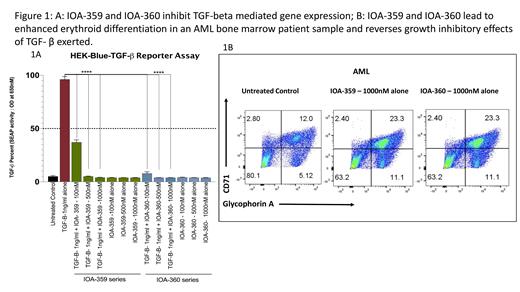
Using the TREEspot™ SCAN Max Kinase Panel of 403 kinases, IOA-359 and IOA-360 were found to be highly selective for ALK5. Both, IOA-359 and IOA-360 have a blocking activity against ALK5 similar to clinical stage ALK5 inhibitors. To evaluate the efficacy of IOA-359 and IOA-360, we first used HEK 293- Blue-TGF-β cells that stably overexpresses a SMAD inducible secreted embryonic alkaline phosphatase (SEAP) reporter. Both IOA-359 and IOA-360 were able to significantly inhibit TGF-β driven reporter at nanomolar doses ( Fig1A). Next, we showed that both ALK5 inhibitors were able to block TGF-β-mediated SMAD2/3 downstream phosphorylation/activation in a dose dependent manner in MDS and leukemic cell lines. Functionally, the ALK5 inhibitors did not lead to any increase in viability or proliferation in leukemic cell lines.
We next tested the efficacy of the novel ALK5 inhibitors in primary patient samples from MDS and AML (N=8). Treatment with both ALK5 inhibitors led to increase in erythroid colony formation in majority of MDS samples in clonogenic assays. Colonies were picked up and examined for erythroid and myeloid differentiation by FACS analysis. We observed that ALK5 inhibition led to increase in Glycophorin A and CD71 expression suggesting enhanced erythroid differentiation ( Fig1B). Myeloid differentiation effects were modest in comparison. Furthermore, in selected samples, addition of Luspatercept led to greater maturation of erythrocytes. Treatment of healthy CD34 + hematopoietic stem and progenitors did not lead to significant changes in differentiation. Lastly, to evaluate downstream effects of ALK5 inhibition, we performed RNAseq analysis in MDS cells. Inhibition with ALK5 inhibitors led to numerous changes in genes affecting cellular differentiation, validating functional changes observed by us.
Conclusion:
Our current results support the preclinical in vitro efficacy of ALK5 inhibitors IOA-359 and IOA-360 alone and in combination with Luspartercept, highlighting their potential for further development and clinical testing in MDS/AML.
Disclosures
Zhao:Albert Einstein COM: Current Employment. Shastri:Kymera Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Rigel Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Gilead Sciences: Membership on an entity's Board of Directors or advisory committees. Lahn:iOnctura: Current Employment, Current equity holder in private company. Verma:Acceleron: Consultancy; Novartis: Consultancy; Eli Lilly: Research Funding; Medpacto: Research Funding; Incyte: Research Funding; GSK: Research Funding; Curis: Research Funding; Janssen: Honoraria; Bakx: Consultancy, Current equity holder in private company; Stelexis: Consultancy, Current equity holder in private company, Honoraria; Throws Exception: Current equity holder in private company; Celgene: Consultancy; Bristol Myers Squibb: Research Funding; Prelude: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal