Background: The combination of daratumumab (D), pomalidomide (P), and dexamethasone (d) has previously demonstrated high overall response rates in patients with RRMM. Results of the phase 3 APOLLO study of DPd vs Pd in patients with RRMM with at least 1 prior line of therapy showed 69% ORR with a median PFS of 12.4 months in those patients who received the triplet combination (Dimopoulos, et al. Lancet 2021). Quadruplet regimens may further improve results by providing deeper and more durable responses with more intensive regimens. We report results from a phase 2 multicenter trial of the addition of Ixazomib to DPd (DIPd) in patients with early RRMM.
Methods: This is a prospective, multi-center, open-label, single arm phase 2 study (NCT03590652) with a primary endpoint of overall response rate (ORR) and safety of DIPd. Secondary endpoints include progression-free survival (PFS), overall survival (OS), and MRD-negativity rate. A Simon's optimal 2-stage design was used, with 14 subjects in stage 1 and 32 patients for stage 2. Eligible patients may not have had prior exposure to daratumumab or Ixazomib, may not have progressed on prior pomalidomide, and must have received ≥1 or ≤3 prior lines of therapy including lenalidomide and a proteasome inhibitor. The first six patients in a safety run-in received daratumumab 16mg/kg IV weekly x 8 doses, biweekly x 8 doses, then monthly, pomalidomide 4mg orally on days 1-21, ixazomib 4mg orally on days 1, 8, 15, and dexamethasone 20-40mg weekly on a 28-day cycle. Grade 3-4 neutropenia was observed in 100% of patients in the safety run-in, prompting starting dose reductions to ixazomib 3 mg and pomalidomide 3 mg by the DSMB for subsequent patients. An amendment allowed for subcutaneous (SC) Daratumumab administration and all active patients transitioned to or started with SC dosing for ongoing therapy. MRD assessments were performed by EuroFlow for patients in suspected CR. Pharmacodynamic changes in patients' tumor microenvironments were established by custom panel mass cytometry to include T-cell memory and activated subpopulations, B-cell content, NK-cell subpopulations as well as MDSCs, Tregs and T-exhaustive markers, monocytes and dendritic cells.
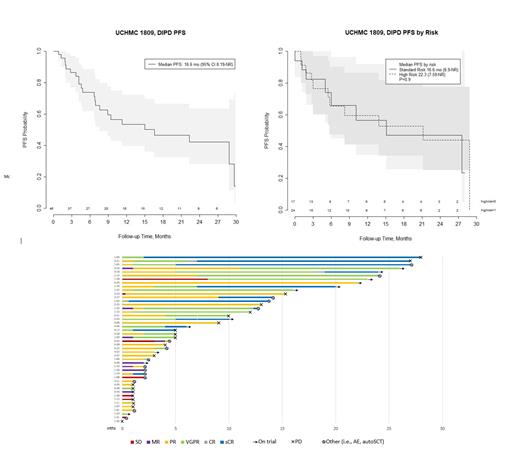
Results: The study has completed the planned enrollment of 46 patients. The median age is 61.6 years (range 41-87), with 47% female, 72% White, 17% Asian, and 5% Black patients. Patients had a median of 1 prior line of therapy (range 1-3), and 56% (23/41) of patients had at least one high-risk cytogenetic abnormality including del 17p, +1q, t(14;16), t(14;20), or t(4;14). Grade 3-4 treatment emergent adverse events (AE) include neutropenia (72%), infection (19%), febrile neutropenia (8%), lymphopenia (8%), electrolyte disturbance (6%), leukopenia (3%), thrombocytopenia (3%), respiratory condition (3%), infusion reaction (3%), thrombosis (3%), and psychiatric condition (3%). ORR to date is 83% (38/46) in evaluable patients, and best responses include 12 (26%) sCR, 12 (26%) VGPR, and 14 (30%) PR. The median duration of response (DOR) is 21.1 months for all patients, with 11 patients currently remaining on DIPd therapy, and 9 deaths all unrelated to study treatment (5 progressive disease [PD], 2 sepsis, 1 COVID, 1 unrelated surgical complication). Of the 35 patients off therapy, 20 patients had PD, 6 patients had AEs, 5 patients stopped per physician discretion or withdrew consent, and 4 patients received an autologous stem cell transplant. After a median follow up of 15 months, the median PFS is 16.6mo (95% CI: 8.19-NR ) for all patients, 16.6mo (6.9-NR) for standard risk patients, and 22.3mo (7.6-NR) for high risk patients (p=0.9). The median OS for all patients is 30.3mo (95 CI: 30.3-NR). Data will be updated for the conference to include MRD negativity rates and completed data analysis of the primary endpoint.
Conclusion: The quadruplet regimen DIPd shows that the addition of a 4 th agent results in high overall response rates and early PFS rates, with manageable toxicity, and the convenience of a nearly all-oral drug regimen in patients with early RRMM. These data are particularly compelling for high-risk disease as more than half of the patients treated had at least one high-risk cytogenetic abnormality, offering a promising option for the management of early relapse in high-risk patients.
Disclosures
Larson:TORL Biotherapeutics: Current equity holder in private company; Pfizer: Research Funding; ImmPACT Bio: Research Funding; Novartis: Research Funding; Bioline: Research Funding; Ionis: Research Funding; Janssen: Research Funding; Bristol Myers Squibb: Research Funding; Allogene: Research Funding; 1200 Pharma: Current equity holder in private company; AbbVie: Research Funding. Costello:Takeda: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Regeneron: Honoraria; Poseida, Ionis, Harpoon Therapeutics: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal