Background: Although many new treatment options have become available, multiple myeloma (MM) is still considered an incurable disease. The importance of achieving minimal residual disease (MRD) negativity in MM patients has become clearer in recent years. Multiple studies show that bone marrow based MRD assessment is one of the strongest prognostic factors, and deeper responses correlate with more favorable outcomes.
The REMNANT study will evaluate if treating at MRD relapse after first line (1.L) treatment prolongs progression-free survival (PFS) and overall survival (OS) in MM patients.
Methods: The REMNANT study (RElapse from Mrd Negativity As iNdication for Treatment) is an academic, multicenter, open-label, randomized phase 2/3 study of newly diagnosed (ND) MM patients eligible for autologous stem cell transplant (ASCT). The study has a population-based approach with few exclusion criteria, implying that patients with kidney failure, amyloidosis, plasma cell leukemia and other comorbidities are enrolled. To increase enrollment in part 2, patients who have received 1.L treatment outside the REMNANT study can be enrolled directly if they are >CR/MRD negative.
391 NDMM patients (age 18-75 years) eligible for ASCT will be enrolled in part 1 (phase 2) of the study and receive standard of care Norwegian 1.L treatment; 4 pre-transplant induction and 4 post-transplant consolidation cycles of bortezomib, lenalidomide and dexamethasone (VRd). After induction, patients will undergo tandem or single transplant, depending on toxicity and response to first transplant. All patients receive lenalidomide maintenance.
Following 1.L treatment 176 patients achieving MRD negative (Euroflow NGF 10 -5) complete response will be enrolled in part 2 (phase 3) of the study. Patients will be randomized in a 1:1 ratio to receive second line treatment (2.L) at MRD relapse in arm A or at PD in arm B. At loss of MRD negativity in arm A or at PD in arm B, 2.L treatment will be daratumumab, carfilzomib and dexamethasone until PD.
The primary endpoint of part 1 (phase 2) of the study is the number of patients who achieve MRD negative (Euroflow NGF 10 -5) complete response 30-45 days post consolidation. Secondary endpoints includes response rates, safety evaluations and patient-reported outcome.
The co- primary endpoints in part 2 (phase 3) are PSF and OS from randomization to progressive disease or death on 2.L treatment. We will also compare different methods for measuring MRD: NGF Euroflow, Next Generation Sequencing, mass spectrometry and PET-CT. We want to evaluate how the different methods compare when it comes to sensitivity and outcome (PFS and OS).
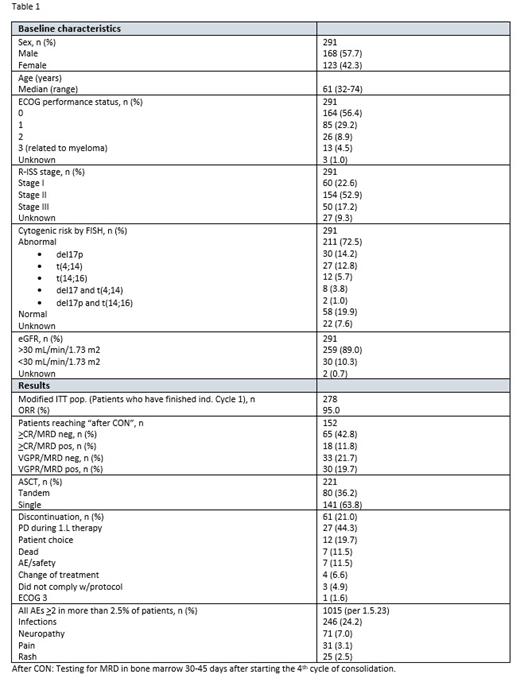
Results: As of July 18 th 2023, 291 patients are enrolled in part 1 and 97 in part 2 (23 directly enrolled). Baseline characteristics, safety summary and preliminary results can be found in Table 1. The ORR on first line treatment is 95% and 65 of 152 patients (45%) were >CR and MRD negative at the post-consolidation visit. Patients achieving > VGPR but not CR MRD negativity post consolidation are followed for up to 24 months on lenalidomide maintenance, and 11 of 74 patients (15%) in follow-up have converted to >CR/MRD negativity at a median time of seven months (range 2-16).
Nine patients have started 2.L treatment in part 2 (phase 3), eight in arm A (MRD guided) and one in arm B (control arm). Median time to loss of MRD negativity among the eight patients in arm A was four months (range 1-6).
The most common adverse events were infections, occurring in 24% of patients (any grade).
During 1.L treatment, 61 patients have left the study. Seven patients have died, 27 discontinued due to disease progression, 12 patients have withdrawn from the study and seven discontinued due to adverse events. 59% of the patients who progressed during 1. L treatment had high-risk cytogenetics (with gain1q and/or del17p and/or t(4;14)).
Conclusion: Standard of care Norwegian first line treatment has an ORR of 95%. The MRD negativity rate among patients who have finished 1.L treatment was 43% (65 of 152). The median time to loss of MRD negativity in arm A (MRD guided) was 4 months.
Disclosures
Askeland:Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria; Janssen-Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees. Eilertsen:Janssen-Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bayer: Honoraria, Membership on an entity's Board of Directors or advisory committees. Moksnes:Abbvie: Honoraria. Tsykunova:Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen-Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Ablynx: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees. Peceliunas:Johnson & Johnson: Consultancy, Other: Travel expenses; Amgen: Consultancy, Other: Travel expenses. Slørdahl:Janssen-Cilag: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy; GSK: Consultancy; Amgen: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Takeda: Honoraria. Schjesvold:Bristol Myers Squibb: Consultancy, Other: Honoraria for lectures and educational material; Amgen: Other: Honoraria for lectures and educational material; Novartis: Other: Honoraria for lectures and educational material; GlaxoSmithKline: Consultancy, Honoraria, Research Funding; Targovax: Research Funding; Celgene: Consultancy, Other: Honoraria for lectures and educational material, Research Funding; Oncopeptides: Consultancy, Other: Honoraria for lectures and educational material, Research Funding; Takeda: Consultancy, Other: Honoraria for lectures and educational material; Janssen-Cilag: Consultancy, Other: Honoraria for lectures and educational material, Research Funding; Pfizer: Other: Honoraria for lectures and educational material; Sanofi: Consultancy, Other: Honoraria for lectures and educational material, Research Funding; Abbvie: Consultancy, Other: Honoraria for lectures and educational material; Skylite DX: Other: Honoraria for lectures and educational material; Daiichi Sankyo: Other: Honoraria for lectures and educational material; Schain: Other: Honoraria for lectures and educational material.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal