Background : Available just one year after the beginning of the pandemic, in December 2020, vaccination has been a revolution allowing to avoid severe forms of the deadly COVID-19 infectious disease in the healthy population as well as in immunocompromised hosts, including recipients of allogeneic stem cell transplantation (Allo-SCT). These patients (pts) were first vaccinated after transplant but rapidly both pts and donors could receive vaccine before Allo-SCT. The impact of vaccination pre- or post- Allo-SCT or both has not been clearly evaluated yet. Moreover, the role of a previous COVID-19 infection before Allo-SCT remains to be specified.
Methods : The impact of pre- and post-Allo-SCT COVID-19 vaccination and previous COVID-19 infection in adults allografted in our Hematology Department between October 2020 and March 2023 and with a follow-up of at least 3 months post-transplant was investigated. Data were collected up to July 2023 and pts or donors were joined by phone if needed to ensure updated information. Three groups were defined : 1) pts or donors with no vaccine and no previous COVID-19 infection before Allo-SCT and vaccination after transplant, 2) pts and/or donors vaccinated and without COVID-19 infection before transplant (with or without vaccine after transplant), and 3) pts and/or donors with a previous COVID-19 infection (with or without vaccine pre and post-transplant). The rates of overall and severe forms of COVID-19 infections after Allo-SCT were collected overall and in each group. Severe forms were defined by COVID-19-related hospitalizations (including those in intensive care unit) and/or death. Some pts also received tixagevimab and cilgavimab (Tix/cil) as preventive treatment after Allo-HSCT during the Delta wave.
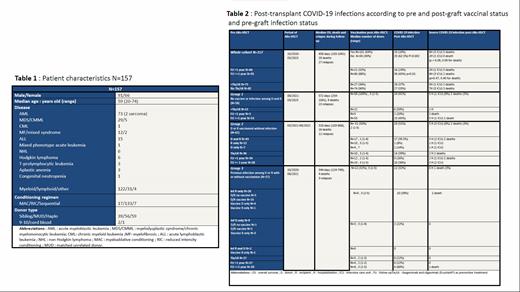
Results : A total of 157 pts was considered (Table 1.median age : 59 yo, range : 20-74 ; male n=91; myeloid disease n=122 ; lymphoid disease n=31; other disease n= 4; conditioning regimen : myeloablative n=17 ; reduced-intensity n=133 ; sequential n=7 ; donor type : sibling n=39, matched unrelated n=56, haploidentical n=59, one mismatch unrelated n=2, cord blood n=1). Post-Allo-HSCT vaccination rates were 100% in group 1 (n=58/58), 50% in group 2 (n=31/62) and 32% (n=12/37) in group 3. Most pts were considered during the delta/omicron waves. The first vaccination post-graft was performed at a median of 168 days (range: 27-482) while COVID-19 infection occurred at a median of 312 days (range: 0-1027) post-graft. Results are summarized in Table 2.
Overall and severe COVID-19 infection rates were 35% and 9.5%, respectively while there were 3% of COVID-related deaths for the whole cohort. Post-Allo-HSCT vaccination was clearly associated with protection against COVID-19 infection (incidence of 20% in vaccinated pts vs 62.5% in non-vaccinated, p<0.001). However, rates of severe forms or deaths related to COVID-19 were similar between vaccinated and non-vaccinated pts.
COVID-19 infection rates were 24%, 31% and 32% in groups 1, 2 and 3, respectively. Severe COVID-19 infection and death rates were also similar between the three groups at 12%, 11% and 3%, and 3%, 3% and 3%, respectively. Considering only pts vaccinated after transplant, all rates were again similar between each group, suggesting that pre-graft vaccination or infection before Allo-HSCT do not provide more protection.
Interestingly, pts in the first year post-transplant had no superior risk of COVID-19 infection or severe forms of the disease. Tix/cil was used mainly in non-vaccinated post-transplant patients and was associated with the same rate of COVID-19 infections compared to patients not receiving Tix/cil. As these latter patients were almost all vaccinated after transplant (90%), this confirms that Tix/cil was as efficient as vaccine use during the Delta wave to protect patients who did not receive vaccine after transplant. Deaths and relapse rates were also comparable between the three groups suggesting no impact of pre-Allo-SCT vaccine or infection on outcomes.
Conclusion : In this study, post-Allo-SCT vaccination appears to be of crucial importance to protect recipients against COVID-19 infections. Pre-graft vaccine or infection are not associated with less COVID-19 infections nor severe forms of the disease in pts vaccinated after transplant, suggesting that post-graft vaccination is sufficient to ensure good protection in these pts.
Disclosures
Chevallier:Incyte: Honoraria, Research Funding; Sanofi: Honoraria; Mallinckrodt Pharmaceuticals: Honoraria; Takeda: Honoraria; Immedica Pharma: Honoraria; Servier: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal