Background & Significance:
Allogeneic stem cell transplantation (allo-SCT) is the curative treatment for transfusion dependent thalassemia (TDT), until the gene therapy is readily accessible, which could revolutionize the treatment approach. 1 Later, Mathews et al. identified additional factors affecting outcomes, leading to the recognition of a specific very high-risk subgroup within Class III, referred to as Vellore high risk (HR) or Class IIIB. 2, 3 Following the identification of Class IIIB, treosulfan (Treo) was introduced as a treatment option due to its favorable low hepatic toxicity profile. 4, 5 Several studies have reported the use of Treo-based conditioning in the high-risk subset, showing treatment-related mortality (TRM) rates ranging from 7 to 21%. 6 - 8 Here, we present our (allo-SCT) experience for transfusion-dependent thalassemia (TDT) using the Flu/Bu/Cy/rabbit ATG or Flu/Thio/Treo as a conditioning regimen in a high risk cohort.
Study design & Methods:
We did a retrospective analysis of all transfusion dependent thalassemia [TDT] patients who underwent allogeneic stem cell transplant [allo-SCT] with Flu/Bu/Cy/ATG or Flu/Thio/Treo as a conditioning regimen. TDT patients who have received conditioning other than this were excluded from the study. The objective of the study is the assess the rate of thalassemia free survival [TFS], thalassemia GVHD free survival [TGFS], overall survival [OS], graft rejection and non-relapse mortality [NRM].
Results:
We included a total of 64 patients in our study. Forty-one patients in Flu/Bu/Cy/ATG group and 23 patients were in Flu/Thio/Treo group.
The median age of the patients was 9 years (range: 8 -14) and 10 years (8 - 15) in Flu/Bu/Cy/ATG and Flu/Thio/Treo group respectively. Upon presentation, the distribution of patients among Class I, II, and III were 2(4.9%) v/s 1(4.4%), 27(65.8%) v/s 11(47.8%), and 12(29.3%) v/s 11(47.8%) respectively. The graft source was bone marrow in 38 (92.7%) patients all in Flu/Bu/Cy group, whereas 3 (7.3%) v/s 23(100%) in peripheral blood stem cells. The mean CD34 stem cell dose administered was 3.4 × 10^6/kg (range: 0.7 - 9.8) v/s 5 × 10^6/kg (1.65 - 6.1). Neutrophil and platelet engraftment occurred at a median of 17 days (range: 11 - 26) v/s 15.5 days (range: 12 - 20) and 18 days (11 - 47) v/s 15 (9 - 41) respectively. Table:1
Primary rejection was in 3 (7.3%) whereas 2 (4.8%) were secondary rejection in Flu/Bu/Cy group only. Mixed chimerism was observed in a higher percentage of patients in the Flu/Bu/Cy/ATG group 17 (41.5%) compared to the Flu/Thio/Treo group 4 (17.4%). Veno-occlusive disease was reported in 14 (34.1%) v/s 4 (17.4%) patients, with [6 (14.6%) v/s 0] cases being mild, [6 (14.6%) v/s 3 (13%)] cases moderate, and [2 (4.8%) v/s 1 (4.3%)] case severe.
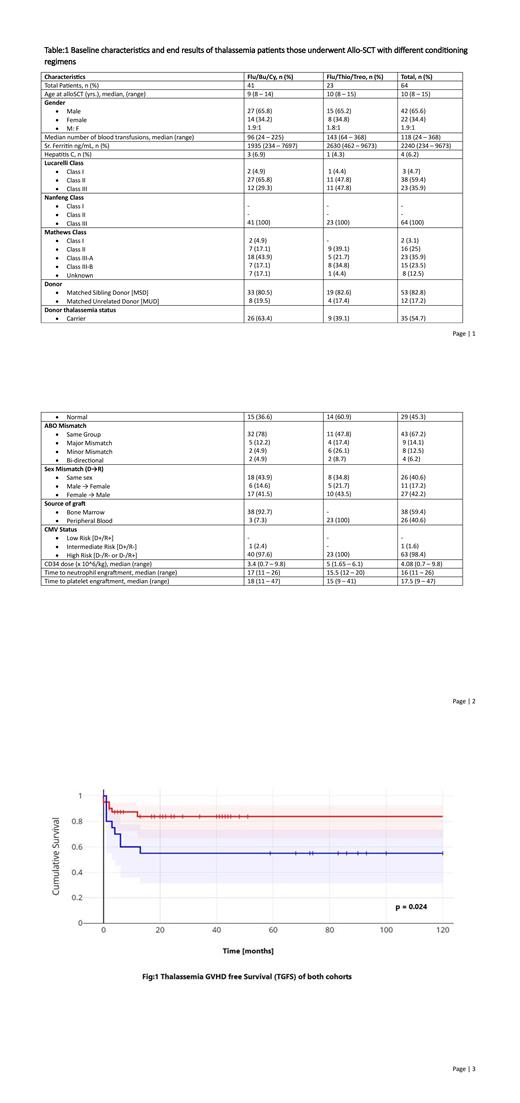
Acute and Chronic graft-versus-host disease occurred in patients [7 (17.1%) v/s 10 (43.5%)] and [1 (2.4%) v/s 7 (30.4)] patients respectively. Fortunately, there were no cases of treatment-related mortality in Flu/Bu/Cy/ATG whereas 3 (13.3%) TRM was observed in Flu/Thio/Treo group. The overall survival at a median follow-up of 25 months were 100% v/s 87% [CI: 64.8 - 95.6] was statistically significant (p=0.02). Thalassemia GVHD free survival at a median follow-up was highly significant with a p value of 0.024 {83.7% [CI: 66.9 - 92.5] v/s 55% [CI: 31.3 - 73.5] (p = 0.024)} in Flu/Bu/Cy and Flu/Thio/Treo group respectively. Fig:1
Conclusion:
In conclusion, the study suggests that the Flu/Bu/Cy/ATG conditioning regimen resulted in better overall survival [OS] and thalassemia GVHD free survival [TGFS] compared to Flu/Thio/Treo regimen in a high-risk transfusion dependent thalassemia [TDT] patients undergoing allo-SCT. However, it is essential to consider the limitations of the study, such as its retrospective nature and the relatively small sample size, before making any definitive conclusion. Further research with large sample sizes and prospective study designs may be needed to confirm these finding.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal