Background: Due to challenges of factor (F)VIII administration, many infants with severe hemophilia A (HA) do not receive prophylaxis until ≥1 year of age. Emicizumab can be administered subcutaneously from HA diagnosis, which may reduce risk of spontaneous and traumatic bleeds, including intracranial hemorrhage. This primary analysis of HAVEN 7 (NCT04431726) evaluates the efficacy, safety, and pharmacokinetics (PK) of emicizumab in infants ≤12 months of age with severe HA without FVIII inhibitors.
Methods: HAVEN 7 is a Phase IIIb, multi-center, open-label study. Informed consent from parents/caregivers and ethics approval have been obtained. Participants received subcutaneous emicizumab 3mg/kg weekly for 4 weeks, then every 2 weeks for 52 weeks; for the 7-year long-term follow-up, participants could continue on this dosing regimen or switch to either 1.5mg/kg weekly or 6mg/kg every 4 weeks. Efficacy endpoints include treated bleeds, all bleeds, treated spontaneous bleeds, and treated joint bleeds. Annualized bleed rates (ABRs) are estimated using a negative binomial regression model. Safety endpoints include adverse events (AEs), thromboembolic events (TEs), thrombotic microangiopathies (TMAs), and immunogenicity (anti-emicizumab antibodies [ADAs] incidence and de novo FVIII inhibitors development). PK endpoints include plasma trough emicizumab concentrations.
Results: At the primary analysis clinical cut-off date (CCOD; May 22, 2023),55 participants
had received emicizumab for ≥52 weeks (median [range] treatment duration: 100.3 [52-118] weeks); all were male, with median (range) age at enrollment of 4.0 months (9 days-11 months; 45.5% [n=25] <3 months; 54.5% [n=30] ≥3-≤12 months), and age at CCOD of 29.0 (12-39) months. Prior to the study, 30 (54.5%) were minimally treated (≤5 exposure days), and 25 (45.5%) were previously untreated participants (PUP).
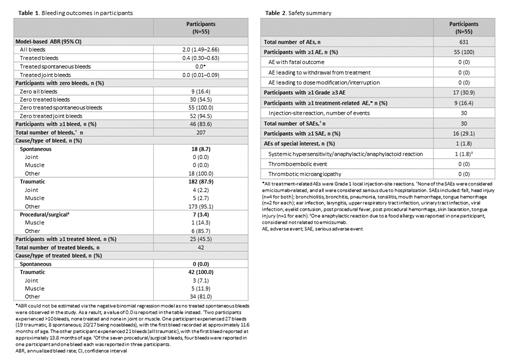
Model-based ABR (95% confidence interval [CI]) for treated bleeds was 0.4 (0.30-0.63), with 54.5% of participants (n=30) having zero treated bleeds ( Table 1). ABRs for all bleeds and treated joint bleeds were 2.0 (95% CI: 1.49-2.66) and 0.0 (0.01-0.09), respectively.
No intracranial hemorrhage occurred. Overall, 207 bleeds (treated or untreated) were reported in 46 participants (83.6%), 87.9% traumatic ( Table 1). In total, 42 treated bleeds, all traumatic, were reported in 25 participants (45.5%). No participant had >3 treated bleeds. Thirty-seven participants (67.3%) had 0-3 bleeds, and 9 (16.4%) had no bleeds. Two participants had >10 bleeds, all untreated and none in joint/muscle: 27 bleeds in one participant (19 traumatic, 8 spontaneous), and 21 in the other (all traumatic).
Emicizumab dose was up-titrated from 3mg/kg biweekly to weekly in one participant per investigator request based on decreasing emicizumab levels measured locally. The participant experienced three treated and two untreated bleeds (all traumatic) before and after up-titration, respectively.
All 55 participants had ≥1 AE, and 9 (16.4%) had ≥1 emicizumab-related AE (all Grade 1 injection-site reaction). No AE led to treatment changes or withdrawal ( Table 2). Sixteen participants (29.1%) reported 30 serious AEs (SAEs), all considered serious due to hospitalization, none considered emicizumab-related. An anaphylactic reaction was reported in one participant (1.8%) following food allergy, not considered emicizumab-related. No deaths, TEs, or TMAs occurred.
Evaluable PK data in 55 participants showed mean trough emicizumab concentrations increased during loading, with values of 62.0µg/mL (95% CI: 58.3-65.6) at Week 5. Thereafter, steady-state concentrations were sustained at 57-66µg/mL. Emicizumab concentrations were higher than those with the same dosing regimen in the HAVEN 2 and 3 studies (46-48µg/mL None of the 55 participants tested positive for ADAs at any timepoint (baseline, Week 5 or 12-weekly thereafter). One PUP was confirmed positive for FVIII inhibitors on Day 603 after three FVIII exposure days (traumatic bleeds treatment). At the CCOD, confirmatory titer was pending for another PUP testing positive for FVIII inhibitors on Day 428, after 10 FVIII exposure days (post tonsillectomy bleed management).
Conclusions: This primary analysis of HAVEN 7 confirms that emicizumab is efficacious and well tolerated in infants with severe HA without FVIII inhibitors. PK profiles were similar to previous studies in adult populations.
Disclosures
Pipe:Takeda: Consultancy; Sanofi: Consultancy; Roche/Genentech: Consultancy; Regeneron/Intellia: Consultancy; Pfizer: Consultancy; Novo Nordisk: Consultancy; LFB: Consultancy; Freeline: Consultancy; HEMA Biologics: Consultancy; GenVentiv: Consultancy; Equilibra Bioscience: Consultancy; CSL Behring: Consultancy; BioMarin: Consultancy; Bayer: Consultancy; ASC Therapeutics: Consultancy; Apcintex: Consultancy; Spark Therapeutics: Consultancy; uniQure: Consultancy. Collins:HAVEN 7 trial steering committee: Membership on an entity's Board of Directors or advisory committees. Dhalluin:F. Hoffmann-La Roche Ltd: Current Employment, Ended employment in the past 24 months. Kenet:PedNet foundation: Membership on an entity's Board of Directors or advisory committees; Bayer, BioMarin, BPL, CSL, Pfizer, Novo Nordisk, Roche, Sanofi- Genzyme, Sobi, Spark, Takeda, Uniqure: Honoraria; BSF, Opko Biologics, Pfizer, Roche, Shire: Research Funding; ASC Therapeutics, Bayer, BioMarin, Novo Nordisk, Pfizer, Roche, Sanofi- Genzyme, Sobi, Takeda, Uniqure: Consultancy; Sheba Medical Center and Sackler Faculty of Medicine, Tel Aviv University: Current Employment. Schmitt:F. Hoffmann-La Roche Ltd: Current Employment, Current equity holder in publicly-traded company; Anti- FIXa/FX bispecific antibody: Patents & Royalties: Co-inventor of a patent related to an anti- FIXa/FX bispecific antibody. Buri:F. Hoffmann-La Roche AG: Current Employment, Current equity holder in publicly-traded company. Jiménez-Yuste:Pfizer: Consultancy, Honoraria, Research Funding; CSL Behring: Consultancy, Honoraria, Research Funding; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Bayer: Consultancy, Honoraria, Research Funding; Novo Nordisk: Consultancy, Honoraria, Research Funding; BioMarin: Consultancy; Sanofi: Consultancy, Honoraria, Research Funding; Sobi: Consultancy, Honoraria, Research Funding; Grifols: Consultancy, Honoraria, Research Funding. Peyvandi:Sobi: Membership on an entity's Board of Directors or advisory committees; CSL Behring: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Takeda: Speakers Bureau; Spark: Speakers Bureau; Sanofi: Membership on an entity's Board of Directors or advisory committees; Biomarin: Membership on an entity's Board of Directors or advisory committees. Young:CSL Behring: Consultancy, Speakers Bureau; Takeda: Consultancy, Research Funding; Novo Nordisk: Consultancy; Hema Biologics/LFB: Consultancy; Hema Biologics: Speakers Bureau; Genentech/Roche: Consultancy; Sanofi Genzyme: Consultancy, Speakers Bureau; Spark: Consultancy, Speakers Bureau; Genentech, Inc.: Research Funding; Viatris: Patents & Royalties. Oldenburg:Stiftung Hamotherapie-Forschung, Working Group Richtlinien zur Gewinnung von Blut und Blutbestandteilen und zur Anwendung von Blutprodukten (Hamotherapie) of the Scientific Advisory Board of the German Medical Association: Membership on an entity's Board of Directors or advisory committees; Bayer, Biogen Idec, Biomarin, Biotest, Chugai, CSL-Behring, Freeline, Grifols, LFB, Novo Nordisk, Octapharma, Pfizer, Roche, Sanofi, Sparks, Swedish Orphan Biovitrum, Takeda: Speakers Bureau; Bayer, Biogen Idec, Biomarin, Biotest, Chugai, CSL-Behring, Freeline, Grifols, LFB, Novo Nordisk, Octapharma, Pfizer, Roche, Sanofi, Sparks, Swedish Orphan Biovitrum, Takeda: Honoraria; Bayer, Biogen Idec, Biomarin, Biotest, Chugai, CSL-Behring, Freeline, Grifols, LFB, Novo Nordisk, Octapharma, Pfizer, Roche, Sanofi, Spark, Swedish Orphan Biovitrum, Takeda: Other: Reimbursement of travel expenses; University Clinic Bonn: Current Employment; Bayer, Biotest, Chugai, CSL-Behring, Novo Nordisk, Octapharma, Pfizer, Roche, Swedish Orphan Biovitrum, Takeda: Research Funding; Working Group Blood of the Ministry of Health, Bayer, Biogen Idec, Biomarin, Biotest, Chugai, CSL-Behring, Freeline, Grifols, LFB, Novo Nordisk, Octapharma, Pfizer, Roche, Sanofi, Sparks, Swedish Orphan Biovitrum, Takeda: Consultancy. Mancuso:IRCCS Humanitas Research Hospital: Current Employment; Bayer, CSL Behring, Novo Nordisk, Pfizer, Sobi, Sanofi, Biomarin, Octapharma, Roche, LFB, Takeda: Consultancy; CSL Behring, Bayer, Novo Nordisk, Takeda: Research Funding; Bayer, CSL Behring, Novo Nordisk, Pfizer, Sobi, Sanofi, Biomarin, Octapharma, Roche, LFB, Takeda: Honoraria; Bayer, CSL Behring, Novo Nordisk, Pfizer, Sobi, Sanofi, Biomarin, Octapharma, Roche: Speakers Bureau. Kavakli:CSL Behring: Membership on an entity's Board of Directors or advisory committees; BioMarin: Membership on an entity's Board of Directors or advisory committees; F. Hoffmann-La Roche Ltd, Novo Nordisk, Takeda, Pfizer: Consultancy, Honoraria, Research Funding; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees. Kiialainen:F. Hoffmann-La Roche Ltd: Current Employment, Current equity holder in publicly-traded company. Chang:Spark Therapeutics: Current Employment; F. Hoffmann-La Roche Ltd: Current equity holder in publicly-traded company. Lehle:F. Hoffmann La Roche Ltd: Current Employment, Current equity holder in private company. Niggli:F. Hoffmann-La Roche Ltd: Current Employment, Current equity holder in private company. Fijnvandraat:Novo Nordisk: Consultancy, Research Funding; F. Hoffmann-La Roche Ltd: Consultancy; Sanofi: Consultancy; CSL Behring: Research Funding; Sobi: Consultancy, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal