Introduction: Cancer and Cardiovascular disease (CVD) often coexist and CVD is the leading cause of non-cancer mortality in people with cancer. Antithrombotic medications used to manage CVD are associated with increased bleeding risk, particularly in people with cancer. Appropriate management of CVD is therefore essential in individuals with cancer, both to decrease recurrent CVD as well as to prevent bleeding. In this study, we assessed utilization patterns of antithrombotic medications for CVD following a new diagnosis of cancer.
Methods: Among people receiving primary care at the University of Vermont Medical Center from 2010 to 2019, we identified a cohort of 4,010 subjects with baseline CVD, identified from problem lists or billing codes within the first year of follow-up. Exclusion criteria included baseline cancer diagnosis (cancer known at the time of inclusion in the cohort or within the first year of follow-up) or a known history of venous thrombosis. Incident cancer was defined as new diagnosis of cancer after the first year of follow-up and did not include people with non-melanomatous skin cancers. We used the National Cancer Institute list of ICD-9 and ICD-10 codes for different cancer diagnoses. Patient's use of antithrombotics, prescribed or non-prescribed, were captured using computable phenotypes developed from the electronic outpatient medication reconciliation record and validated using chart review. People with baseline CVD who were diagnosed with cancer after the first year of follow up were matched 1:1 by sex and age with people with CVD and no cancer. We used logistic regression to estimate the odds ratio (OR) and 95% confidence intervals (95% CI) of persistent guideline-directed use of antithrombotic medications for 6 months after a new cancer diagnosis versus a matched time-period in those without incident cancer (use of antiplatelets in those with a history of atherosclerotic CVD and use of anticoagulation in those with a history of atrial fibrillation).
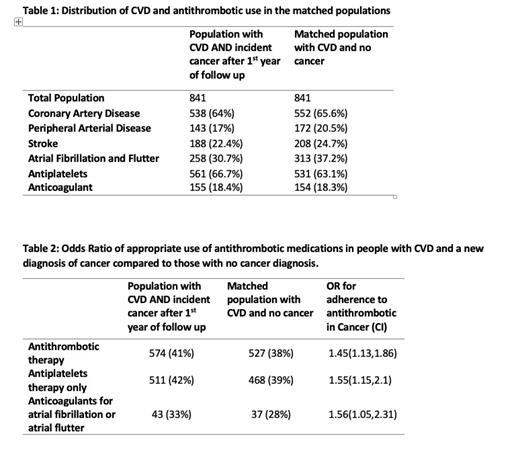
Results: We identified 4,010 individuals with baseline CVD, 841 of whom (20%) developed incident cancer during follow-up. Distribution of CVD and antithrombotic use in the age- and sex-matched population are presented in Table 1. 2/3 of the population had coronary artery disease and about 30% had atrial fibrillation or flutter. About 65% of individuals were on any antiplatelet therapy and 18% were on anticoagulation for CVD. 41% of people with baseline CVD, and incident cancer diagnosis were still on antiplatelet therapy 6 months after their cancer diagnosis as opposed to 38 % in the matched population without a cancer diagnosis. 33% of individuals with incident cancer were still on anticoagulation for atrial fibrillation or flutter as opposed to 28% of the matched population without cancer. Both of these differences were statistically significant with OR=1.55 (95% CI, 1.15-2.1) for antiplatelets, and OR=1.56 (95% CI, 1.05,2.31) for anticoagulants, compared to those with no cancer diagnosis (Table 2).
Conclusion: Surprisingly, our study found that incident cancer in individuals with baseline CVD was associated with increased adherence to antithrombotic therapy. We had hypothesized that incident cancer would be associated with lower use of antithrombotic therapy. This result could be related to increased interaction with medical specialists following a cancer diagnosis. The impact of increased adherence to antithrombotics on bleeding however, will need to be further investigated.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal