Introduction
Anemia can be caused by infections, malignancies, CKD, or chemotherapy. Increasing Hb or fetal hemoglobin (HbF) can prevent or reduce anemia-related pathophysiology. Polycomb inhibitory complex 2 (PRC2), consisting of EZH2, EED, and SUZ12, is a subunit of the major transcriptional corepressor complex of BCL11A, and targeting EED may be one novel approach for treatment of patients with anemia. We report the effects of APG-5918, a small-molecule selective EED inhibitor, on Hb and HbF induction in human CD34⁺ hematopoietic stem cells (HSCs) and the efficacy of APG-5918 in a CKD-induced anemia model in vivo.
Methods
The pharmacodynamic (PD) effects of APG-5918 were evaluated in human cord blood CD34⁺ HSCs by flow cytometry, RT-qPCR, and ELISA. In vivo, the antianemia effect of APG-5918 was evaluated in adenine-induced renal anemic Sprague-Dawley rats.
Results
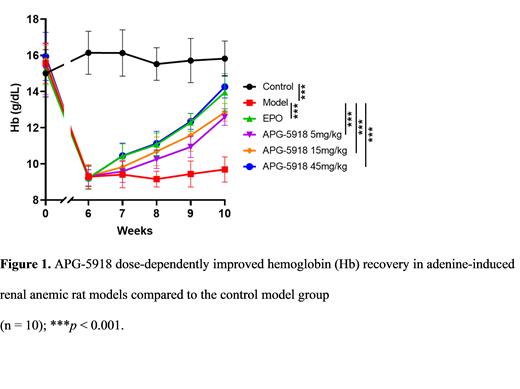
APG-5918 inhibited H3K27me3 inCD34⁺ HSCs, the critical epigenetic mark catalyzed by PRC2. APG-5918 also increased γ-globin coding gene ( HBG) and β-globin coding gene ( HBB) mRNA levels in a dose-dependent manner. After 7 days of treatment with APG-5918 at 1 μM, HBG and HBB mRNA increased to 12.36-fold and 4.76-fold of the control group, respectively, which was pharmacologically superior to effects observed with hydroxyurea at 33 µM (~6.53-fold and 2.27-fold increases in HBG and HBB mRNA, respectively). Hb and HbF protein were also upregulated in a dose-dependent manner. A total of 1.69-fold and 1.9-fold increases in HbF and Hb protein expression were observed after 7 days of treatment with APG-5918 at 1 μM.HbF and Hb protein showed no significant difference after hydroxyurea treatment. In an adenine-induced renal anemic rat model, APG-5918 was administered orally once daily at 3 dose levels (5, 15, and 45 mg/kg). Both body weight and the blood parameters erythrocytes, Hb, hematocrit (HCT), and reticulocytes (RET) improved significantly in a dose-dependent manner compared to the model (adenine) group ( p < 0.05, 0.01, or 0.001) (Figure 1). APG-5918 showed similar in vivo efficacy with erythropoietin (EPO): body weight and blood parameters in the group treated with APG-5918 45 mg/kg were comparable to those in the EPO group. In addition, blood parameters in the APG-5918 (15 mg/kg) plus EPO group recovered to the level of the control group, which was superior to the APG-5918 and EPO monotherapy groups.
Conclusions
APG-5918 demonstrated its effects as a single agent on inhibition of H3K27me3 inCD34⁺ HSCs, HbF and Hb induction, and normalization of hematologic parameters in a CKD-induced anemia model. APG-5918 also showed synergistic antianemia effects when combined with EPO. These findings support APG-5918 as a novel treatment option for CKD-induced anemia.
Disclosures
Zhai:Ascentage Pharma: Current Employment, Current equity holder in publicly-traded company, Other: Leadership (CMO). Yin:Ascentage Pharma: Current Employment, Current equity holder in publicly-traded company. Liang:Ascentage Pharma: Current Employment, Current equity holder in publicly-traded company. Wang:Ascentage Pharma: Current Employment, Current equity holder in publicly-traded company. Min:Ascentage Pharma: Current Employment, Current equity holder in publicly-traded company. Yang:Ascentage Pharma: Current Employment, Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees, Other: Leadership, Patents & Royalties.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal