Background
Rurioctocog alfa pegol is a polyethylene glycol (PEG)-ylated recombinant antihemophilic factor VIII (FVIII) with an extended half-life, which allows prolonged dosing intervals. Using myPKFiT, the first and only FDA-cleared PK dosing software, healthcare professionals can simulate dosing regimens using patients' pharmacokinetic profiles. According to 2020 WFH Guidelines for the Management of Hemophilia, the trough level is suggested to be increased from >1% to 3-5% or higher to improve patient care (Srivastava et al. Haemophilia 2020). The Phase 3b Continuationstudy demonstrated that maintaining trough levels ≥3% with PK-guided prophylaxis in patients with severe haemophilia A reduced spontaneous annual bleeding rate (sABR), and thus led to the resolution of target joints (Chowdary et al. Haemophilia 2020). The ATTRACT-HA study will provide the first multi-center real-world evidence in Taiwan whether maintaining a predicted trough level of ≥3% using rurioctocog alfa pegol PK-guided prophylaxis using myPKFiT in severe hemophilia A patients can result in improved clinical outcomes as well as a better quality of life.This interim analysis will focus on the hemophilia-related characterization of the enrolled patients.
Methods
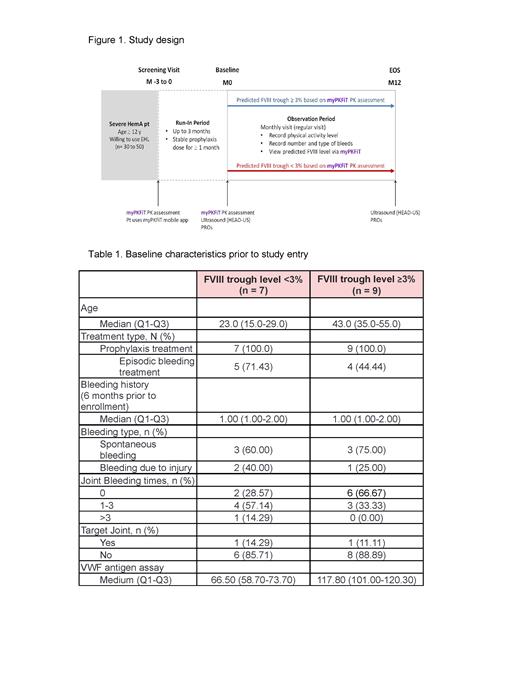
We enrolled patients, ≥12 years old with body weights of ≥30 kg and ≤140 kg, diagnosed with severe hemophilia A (FVIII clotting activity <1%). The enrolled patients were required to be on prophylaxis with rurioctocog alfa pegol for more than a month before the baseline visit and their historical bleeding data, 6 months prior to the screening visit, was also collected. Patients with a stable dosing regimen at the baseline underwent a myPKFiT PK assessment to determine if a patient was in the trough level <3% or ≥3% group (Figure 1). The primary endpoint of this study was to evaluate the clinical outcomes based on ABR and AJBR. Secondary endpoints included the scores of clinical outcomes with haemophilia early arthropathy detection with ultrasound (HEAD-US), the Haemophilia Quality of Life Questionnaire, and the international physical activity questionnaire (IPAQ) assessing patients' daily activity level performed at the baseline visit and in month 12. The date of data cut-off for this interim analysis was 5 th of January 2023.
Results
A total of 16 patients with severe hemophilia A were enrolled and stratified by myPKFiT PK assessment into two groups, with a trough level either <3% or ≥3%. There were 7 patients (median age: 23.0 y/o; Q1-Q3 15-29) in the trough level <3% group and 9 patients (median age: 43.0 y/o; Q1-Q3 35-55) in the trough level ≥3% group. Within 6 months prior to enrollment, 71.43% of the patients in the trough level <3% group experienced episodic bleeding, while only 44.44% of the patients experienced episodic bleeding in the trough level ≥3% group (Table 1). The median baseline HEAD-US score was numerically lower in the trough level <3% compared to the trough level ≥3% group (4.29 vs 24.11). The median score of Haem-A-QoL was 25.93 (Q1-Q3; 16.67-47.28) in the trough level <3% group and 42.93 (Q1-Q3; 28.89-48.37) in the trough level ≥3% group. Prospective 1-year follow-up assessments will be performed thereafter.
Conclusion
The ATTRACT-HA study is the first prospective, multi-center, observational study currently ongoing in Taiwan to provide real-world evidence of the effectiveness of rurioctocog alfa pegol PK-guided prophylaxis using myPKFiT app in patients with severe hemophilia A. In this interim report, we described the clinical features and factors of the study participants at the beginning of this real-world study. Between the two trough levels, we observed a higher percentage of patients experiencing episodic bleeding prior to PK-guided prophylaxis and lower baseline HEAD-US scores in the <3% trough level group, which may be in part due to the younger median age in this group.
Acknowledgments
The authors thank Dr. Brenden Chen for his contribution of clinical insight and editorial support during the abstract development. This study was funded by Takeda Pharmaceuticals Taiwan, Ltd.
Disclosures
Yi-Rou Lei, Chieh-Min Chen, Melody Cheng, Brenden Chen are employees of Takeda Pharmaceuticals Taiwan, Ltd. All the other authors disclose no relevant conflicts of interest.
Disclosures
Lei:Takeda Pharmaceuticals Taiwan: Current Employment. Chen:Takeda Pharmaceuticals Taiwan: Current Employment. Cheng:Takeda Pharmaceuticals Taiwan: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal