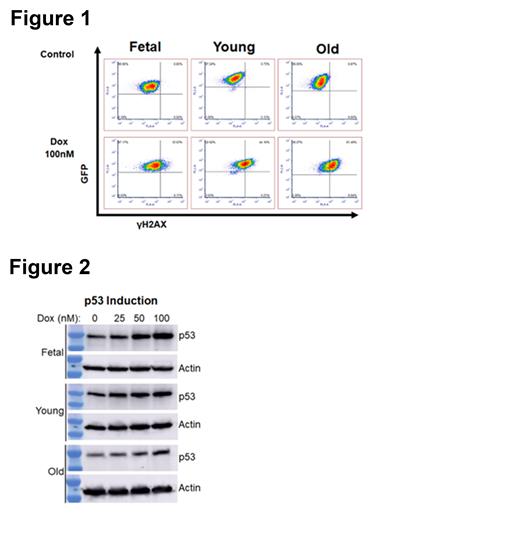
Acute myeloid leukemia (AML) is an aggressive malignancy that leads to the accumulation of immature myeloid precursors, resulting in progressive marrow failure and death. AML arises from the hematopoietic stem cell (HSC), either directly or through an early myeloid precursor. HSCs must survive for the entire human lifespan to provide the 200+ billion blood cells that are produced daily. They are subjected to the entirety of the aging process. Further, while AML is diagnosed in every age range, it disproportionately affects older patients, with a median age at diagnosis of 68. Older age, typically defined as 60 years of age or older, is a powerful, poor prognostic factor. Clinical trials show an estimated 5-year survival of less than 10% in these patients. Ten-year survival is even worse with one study estimating 2.4% of patients over 60 years of age alive at ten years when treated with chemotherapy. Importantly, this poor outcome is the result of disease that is significantly more resistant to therapy. Even though this clinical data is well known the underlying mechanisms of therapy resistance in older AML are unknown. To address this, we generated genetically identical AML cells from fetal, 12-week-old (Young) or 24-month-old (Old) murine HSCs. Retroviral vectors were used to express the oncogenic MLL-ENL fusion protein and NRas G12D. AML was confirmed by morphology, immunophenotype and all AML cells were lethal when injected into syngeneic recipients. Old AML cells demonstrated reduced autophagy, decreased NAD levels and impaired mitochondria consistent with a significant effect of biological aging. When treated with DNA damaging chemotherapy, Old AML cells were significantly more resistant than either Young or Fetal cells to both cytarabine and the anthracycline doxorubicin. To examine the DNA damage response AML cells were treated with doxorubicin and stained for the early DNA damage marker gH2AX. Old AML cells generated significantly more gH2AX than either Fetal or Young AML cells (Figure 1). We next examined the induction of p53 in response to treatment and in contrast to the gH2AX results Old AML cells induced the least amount of p53 in response to doxorubicin treatment (Figure 2). This data demonstrates that Old AML cells were more adept at recognizing DNA damage but least likely to induce p53 in response to it suggesting a difference in DNA repair. To better understand these differences RNA sequencing was performed and differentially expressed genes (DEGs) identified. When Fetal and Young AML cells were compared, no DEGs reached the false discovery threshold for significance, demonstrating that Fetal and Young AMLs have highly similar gene expression profiles. In contrast, when Old AML cells were compared to either Young or Fetal cells, hundreds of highly significant DEGs were identified, and KEGG pathway enrichment analysis revealed that several DNA repair pathways were significantly upregulated in Old AML. These include base excision repair (FDR p= 8.60E-06), mismatch repair (FDR p=3.26E-05), and nucleotide excision repair (FDR p= 0.00043). To further validate these pathways, the Beat AML TCGA dataset was assessed for the prognostic effect of increased expression of key genes in these pathways. Patients with increased expression of these genes had a trend toward worse survival (p=0.07) consistent with these pathways being clinically relevant. These data suggest that AML arising in older patients may overexpress DNA damage repair pathways leading to chemotherapy resistance and poor outcomes. Additional work on ways to increase sensitivity to Old AML cells is in progress.
Disclosures
Pardee:Cornerstone Pharmaceuticals: Consultancy, Research Funding; AbbVie Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Genentech Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal