Background: In a meta-analysis of 5 trials, the addition of gemtuzumab ozogamicin (GO) to intensive induction chemotherapy led to a survival benefit in patients with core-binding factor (CBF) acute myeloid leukemia (AML). The survival benefit was observed with a lower dose or fractionated schedule of GO. Given the heterogenous incorporation of GO in clinical trials, the ideal dose and schedule remains unclear. We report our outcomes of adding a single-dose of GO to intensive induction chemotherapy for patients with CBF-AML.
Methods: We conducted a retrospective analysis to compare outcomes of patients with CBF-AML treated with intensive induction chemotherapy, with and without GO, at the University of Alabama at Birmingham (UAB) between January 2010 and April 2023. Single-dose GO was given at 3mg/m 2 during induction only.
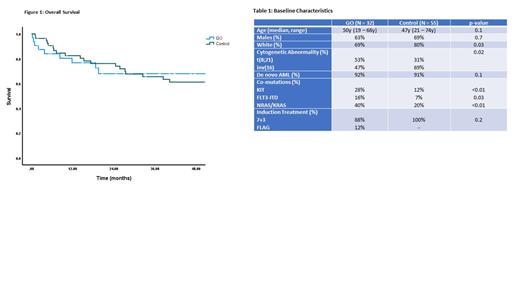
Results: There were 87 patients included in the analysis (GO=32, control=55). The baseline characteristics are shown in Table 1. The median age of the GO and control groups was 50y (19-68y) and 47y (21-74y), respectively. There was an overrepresentation of white patients in the control group. In the GO group, more patients had t(8;21) (53% vs. 31%, p=0.02), KIT (28% vs. 12%, p<0.01), FLT3-ITD (16% vs. 7%, p=0.03) and NRAS/KRAS mutations (40% vs. 20%, p<0.01) compared to the control group. Induction chemotherapy consisted of 7+3 in 88% of patients receiving GO and all patients in the control group. Patients <65y received idarubicin 12mg/m 2 while older patients received daunorubicin 45mg/m 2.
The composite complete remission (cCR) rate was higher in the control group (93%) compared to the GO group (82%) (p<0.001). The rate of measurable residual disease (MRD) negative cCR, by flow cytometry, was similar between both groups (GO=62%, control=69%, p=0.5). There were no significant differences between the two groups in terms of time to neutrophil and platelet recovery, veno-occlusive disease, febrile neutropenia and length of hospital stay.
GO was not administered during consolidation chemotherapy. The 3-year relapse-free survival (RFS) for both groups was similar (71% vs 68%, p=0.5). The 3-year overall survival (OS) for the GO group was 68%, compared to 66% for the control group (p=0.9) (Figure 1).
Conclusion: In our report, we find that survival of patients with CBF-AML is favorable in the real-world setting. These outcomes are comparable to those reported in the meta-analysis leading to the approval of GO. The addition of single-dose GO did not lead to a higher remission rate or survival benefit, when compared to intensive chemotherapy without GO. However, the analysis is limited by differences in baseline characteristics between the two groups with more patients in the GO group harboring high-risk features such as the presence of t(8;21), KIT, FLT3-ITD and KRAS/NRAS mutations. Further investigation into the incorporation of GO in the treatment algorithm for CBF-AML is needed.
Disclosures
Bachiashvili:University of Alabama at Birmingham: Current Employment. Vachhani:Incyte, CTI BioPharma Corp, Blueprint Medicines: Speakers Bureau; Abbvie, Amgen, Blueprint Medicines, Cogent Biosciences, Incyte, CTI BioPharma Corp, Daiichi Sankyo, GlaxoSmith Kline, Karyopharm, Novartis, Pfizer, Genentech, Inc., Servier, Stemline, MorphoSys, LAVA therapeutics: Honoraria. Jamy:Ascentage: Other: Advisory Board Participation.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal