Background: Mosunetuzumab (Mosun) is a CD20xCD3 T-cell engaging bispecific antibody that engages and redirects T cells to eliminate malignant B cells. The ongoing Phase Ib/II GO40516 study (NCT03671018) investigates Mosun in combination with polatuzumab vedotin (Pola; M-Pola) in pts with B-cell non-Hodgkin lymphoma (B-NHL). The Phase II expansion phase of the M-Pola regimen (with conventional Mosun and Pola dose and dose schedule), showed an acceptable safety profile and promising anti-tumor activity in pts with R/R B-NHL at interim analysis (Budde et al. ASH 2021). With enrollment complete, we present full dose-expansion cohort results.
Methods: Eligible pts had confirmed R/R LBCL (including diffuse LBCL not otherwise specified, high-grade B-cell lymphoma [HGBCL], transformed follicular lymphoma [tFL], and follicular lymphoma [FL] grade [Gr] 3b) and had received ≥1 prior line of therapy (including an anti-CD20 antibody). Treatment cycles were 21 days. Pola (1.8mg/kg; intravenous [IV]) was administered on Day (D) 1 of Cycles (C) 1-6. Mosun IV was administered with C1 step-up dosing to mitigate cytokine release syndrome (CRS), with the following dose and dose schedule: 1mg on C1D1, 2mg on C1D8, 60mg on C1D15 and C2D1, and 30mg on D1 of C3+. Pts with a complete response (CR) completed Mosun after C8, and pts with stable disease or partial response at the end of C8 continued Mosun for a total of 17 cycles. Primary endpoint was best overall response rate (ORR) by independent review committee (IRC) using Lugano 2014 criteria (Cheson et al. J Clin Oncol 2014). CRS events were reported using ASTCT criteria (Lee et al. Biol Blood Marrow Transplant 2019).
Results: As of April 7, 2023, 98 pts received M-Pola in the dose-expansion cohort. Median age was 68.0 years (range: 20-88), 71.4% were male, 87.8% had de novo DLBCL, 8.2% had tFL, 4.1% had Gr 3b FL, 18.4% had HGBCL (including double/triple-hit lymphoma), 7.1% had bulky disease (≥10 cm), 51.0% had an International Prognostic Index score 3-5, and 85.7% had Ann Arbor stage III/IV disease. The median number of prior lines of therapy was 2 (range: 1-8), 35.7% had prior CAR-T therapy, and 11.2% had prior ASCT. Most pts (80.6%) were refractory to prior anti-CD20 therapy, 58.2% were primary refractory, 77.6% were refractory to their previous therapy, and 26.5% were refractory to CAR-T therapy. The median number of cycles received was 8 for Mosun (range: 1-17) and 6 for Pola (range: 1-6).
Adverse events (AEs) occurring in ≥15% of pts were fatigue (42.9%), neutropenia (28.6%), nausea (26.5%), diarrhea (23.5%), pyrexia (23.5%), headache (21.4%), CRS (18.4%), chills (17.3%), and peripheral sensory neuropathy (16.3%). Gr 3/4 AEs that occurred in ≥5% pts were neutropenia (20.4%) and fatigue (7.1%). Gr 5 AEs occurred in 3 (3.1%) pts, of which 2 were due to COVID-19 pneumonia and 1 was due to pneumonia. None were considered related to treatment. CRS events were observed in 18.4% of pts: Gr 1 n=11, Gr 2 n=4, Gr 3 n=3. All CRS events were resolved by data cut-off. Three pts received tocilizumab, 2 pts had a vasopressor, 2 pts received high flow oxygen, and 2 pts required ICU admission for CRS. Mosun-related neurologic AEs potentially consistent with immune effector cell-associated neurotoxicity syndrome occurred in 5 pts (5.1%); all were Gr 1/2 except 1 Gr 4 encephalopathy event. Treatment-related neuropathy occurred in 25.5% of pts (all Gr 1/2). Eleven pts (11.2%) discontinued treatment due to AEs, of which 5 (5.1%) were treatment-related: 1 (1.0%) due to encephalopathy (Mosun-related) and 4 (4.1%) due to peripheral neuropathy (Pola-related).
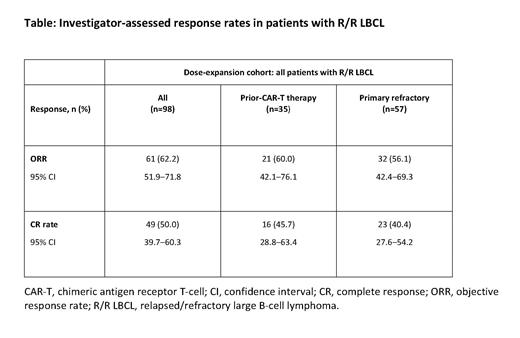
Investigator-assessed ORR and CR rate were 62.2% and 50.0%, respectively ( Table). Median duration of response was not reached (NR; 95% confidence interval [CI]: 11.6-NR); median duration of CR was NR (95% CI: 20.5-NR). Median progression-free survival was 9.6 months (95% CI: 5.6-18.6) and overall survival was 23.3 months (95% CI: 15.2-NR), with a median follow-up of 18.6 months (95% CI: 17.7-23.7). In pts with prior CAR-T therapy (n=35), the ORR and CR rates were 60.0% and 45.7%, respectively. In primary refractory pts (n=57), the ORR and CR rates were 56.1% and 40.4%, respectively.
Conclusions: M-Pola continues to show a favorable safety profile and compelling efficacy in highly refractory pts, including those with prior CAR-T therapy and/or primary refractory disease. Primary analysis data, including IRC responses and biomarker analyses, will be presented.
OffLabel Disclosure:
Budde:AstraZeneca: Consultancy, Research Funding; Roche: Consultancy; MustangBio: Research Funding; Amgen: Research Funding; ADC Therapeutics: Consultancy; Merck: Research Funding; Novartis, Gilead, F. Hoffmann-La Roche Ltd, BeiGene, Genentech, Inc.: Consultancy. Olszewski:Genmab, Blue Cross/Blue Shield of Rhode Island, Schrodinger, ADC Therapeutics, BeiGene: Consultancy; Leukemia & Lymphoma Society, Genetech, Inc. / F. Hoffmann-La Roche Ltd, Adaptive Biotechnologies, Precision Biosciences, Genmab: Research Funding. Assouline:AbbVie: Honoraria; BeiGene: Consultancy; Novartis Canada: Research Funding; Janssen: Honoraria; Roche-Genentech: Honoraria; AstraZeneca: Honoraria; Ipsen: Consultancy; Gilead: Honoraria; Palladin: Honoraria. Lossos:University of Miami: Current Employment; Adaptive: Honoraria; LRF: Membership on an entity's Board of Directors or advisory committees; NCI: Research Funding; NCI: Research Funding; BeiGene: Consultancy. Diefenbach:Genmab. Abbvie, Regeneron, F. Hoffmann-La Roche Ltd / Genentech, Inc., Seattle Genetics, Merck: Membership on an entity's Board of Directors or advisory committees; F. Hoffmann-La Roche Ltd / Genentech, Inc., BMS, Merck, Abbvie, Novartis, Celgene, Cargo, Nekktar: Research Funding; Gillead: Current equity holder in publicly-traded company; OverT Therapeutics: Current equity holder in private company. Kamdar:Genentech, Inc., Celgene for IDMC: Membership on an entity's Board of Directors or advisory committees; Novartis: Research Funding; Seagen: Speakers Bureau; AbbVie, AstraZeneca, Celgene/ Bristol-Myers Squibb, Adaptive Biotechnologies, ADC therapeutics, BeiGene, Genentech, Inc., syncopation, caribou biosciences: Consultancy. Ghosh:Seagen, TG Therapeutics, AstraZeneca, Phamacyclics, Janssen, Bristol Myers Squibb, Gilead Sciences, Kite Pharma, Beigene, Incyte, Lava Therapeutics, Incyte, Roche/Genentech, Novartis, Loxo Oncology, AbbVie, Genmab, Adaptive Biotech, ADC Therapeutics, Morp: Honoraria; AstraZeneca, Janssen, Pharmacyclics, Kite pharma, BMS, Epizyme: Speakers Bureau; Roche NHL solutions panel: Membership on an entity's Board of Directors or advisory committees; TG Therapeutics, Genentech/Roche, Bristol Myers Squibb, Gilead, Morphosys, AbbVie, Pharmacyclics: Research Funding; Seagen, TG Therapeutics, AstraZeneca, Phamacyclics, Janssen, Bristol Myers Squibb, Gilead Sciences, Kite Pharma, Beigene, Incyte, Lava Therapeutics, Incyte, Roche/Genentech, Novartis, Loxo Oncology, AbbVie, Genmab, Adaptive Biotech, ADC Therapeutics: Consultancy. Modi:Karyopharm, ADC Therapeutics, Genentech: Research Funding; Morphosys, Seagen, AstraZeneca (spouse), Genentech (spouse): Consultancy; BeiGene: Speakers Bureau. Sabry:Pfizer (shares as part of my investment bundle): Current equity holder in publicly-traded company; Sitting on Advisory Board for F. Hoffmann-La Roche Ltd, Pfizer, Abbvie, Novartis, Janssen, Incyte: Honoraria. Naik:Genentech, Inc., Abbvie: Research Funding; KPC Therapeutics: Honoraria; Penn State Cancer Institute: Current Employment. Mehta:Astex, BeiGene, Gilead, I-Mab, Incyte/Morphosys, Kite Pharma, Novartis, SeaGen, TG Therapeutics: Consultancy; ADC Therapeutics, Astex, BMS, Celgene, Fate Therapeutics, forty-seven, Gilead, I-Mab, Incyte/Morphosys, Juno Therapeutics, Kite Pharma, SeaGen, TG Therapeutics: Research Funding; Artiva Bioscience, BeiGene, BMS, Celgene, Gilead, Incyte/Morphosys, Kite Pharma, SeaGen: Speakers Bureau. Nakhoda:BMS, BTG/SERB, ADC Therapeutics: Honoraria; BTG/SERB: Research Funding; Fox Chase Cancer Center: Current Employment. Smith:ADC Therapeutics, AstraZeneca, BeiGene, Epizyme, Karyopharm, KITE pharma, Incyte, Numab Therapeutics AG, Abbvie, Coherus Biosciences, advisory board (spouse) Genentech, Inc.: Consultancy; ADC Therapeutics, AstraZeneca, Ayala (spouse), Bayer, BeiGene, Bristol Myers Squibb (spouse), De Novo Biopharma, Enterome, Genentech, Inc., Ignyta (spouse), Incyte Corporation, Kymera Therapeutics, Merck Sharp and Dohme Corp., MorphoSys, Nanjing Pharmaceu: Research Funding; BeiGene: Membership on an entity's Board of Directors or advisory committees. Dorritie:Curio and Dava Oncology: Honoraria; Hoffman-LaRoche: Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Research Funding; Janssen: Research Funding; Kite, a Gilead Company: Research Funding; Genmab: Research Funding. Jia:F. Hoffmann-La Roche Ltd: Current Employment; F. Hoffmann-La Roche Ltd: Current equity holder in publicly-traded company. Pham:F. Hoffmann-La Roche Ltd (Cananda): Current Employment. Huw:F. Hoffmann-La Roche Ltd: Current equity holder in publicly-traded company; Genentech, Inc.: Current Employment. Wu:F. Hoffmann-La Roche Ltd / Genentech, Inc.: Current Employment. To:Genentech, Inc.: Current Employment. Wei:F. Hoffmann-La Roche Ltd: Patents & Royalties; F. Hoffmann-La Roche Ltd: Current equity holder in publicly-traded company; Genentech, Inc.: Current Employment. Chavez:Epizyme: Honoraria; AstraZeneca: Consultancy, Research Funding; Genentech, Inc: Consultancy; Cellectar: Consultancy; TG Therapeutics: Consultancy; Adicet: Consultancy; Novartis: Consultancy; Bristol Myers Squibb: Consultancy; Kite, a Gilead Company: Consultancy; ADC Therapetics: Consultancy, Research Funding; Merck: Research Funding; Lilly: Honoraria, Speakers Bureau; Genmab: Consultancy; Genmab, ADC Therapetics, Kite/Gilead, BMS, Novartis, Adicet, TG Therapeutics, Cellectar, Genentech, Inc., AstraZeneca: Consultancy; Merck, AstraZeneca, Adaptive, Janssen: Research Funding; Lilly, Epizyme, BeiGene: Honoraria; Janssen: Research Funding; BeiGene: Honoraria, Speakers Bureau; Adaptive: Research Funding.
Mosunetuzumab (Lunsumio) is a bispecific CD20-directed CD3 T-cell engager indicated for the treatment of adult patients with relapsed or refractory FL after two or more lines of systemic therapy. Polatuzumab vedotin (Polivy) is a CD79b-directed antibody-drug conjugate indicated: in combination with a rituximab product, cyclophosphamide, doxorubicin, and prednisone (R-CHP) for the treatment of adult patients who have previously untreated DLBCL, NOS or HGBL and who have an IPI score of 2 or greater; and in combination with bendamustine and a rituximab product for the treatment of adult pts with relapsed or refractory DLBCL, NOS after at least two prior therapies.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal